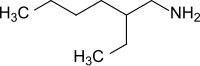
2-ethylhexylamine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Simplified structural formula - mixture of isomers | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-ethylhexylamine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 19 N | |||||||||||||||
| Brief description |
clear, colorless to light yellow liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 129.25 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.79 g cm −3 at 20 ° C |
|||||||||||||||
| Melting point | ||||||||||||||||
| boiling point |
169 ° C |
|||||||||||||||
| Vapor pressure | ||||||||||||||||
| solubility |
slightly soluble in water (2.5 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.4176 (25 ° C ) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Ethylhexylamine (2-EHA) is a sparingly water-soluble primary amine which is derived from the racemic 2-ethylhexanol and whose C 8 -alkyl radical is branched in the 2-position with an ethyl group . Because of its easy accessibility, 2-EHA has a wide range of uses as an amine of medium chain length.
Manufacturing
The amination of 2-ethylhexanal by reaction with hydroxylamine hydrochloride to form the oxime and subsequent reduction with zinc - hydrochloric acid is a simple one-pot method for the preparation of racemic 2-ethylhexylamine.
The amine is also obtained from 2-ethylhexanol by a one-pot reaction with sodium azide in the presence of two-molar triphenylphosphine in the carbon tetrachloride / N , N -dimethylformamide system (1: 4) at 90 ° C via the azide formed as an intermediate in a Staudinger reaction in yields of 85– 95% accessible.
Of industrial interest is the reductive amination when passing 2-ethylhexanol with ammonia in the vapor phase over copper and nickel contacts at temperatures above 200 ° C and short contact times (5-15 sec), which produces a mixture of 2-ethylhexylamine (23 % Yield), di- (2-ethylhexyl) amine (71% yield) and tri- (2-ethylhexyl) amine (2% yield).
An increase in the excess of ammonia and hydrogen leads to a significant shift in the composition of the mixture in the direction of the monosubstitution product 2-ethylhexylamine and to complete hydrogenation of the nitrile formed as an intermediate .
For work-up by distillation, because of the relatively low boiling point difference (2-EH-amine 169 ° C.), conversion of the 2-EH-OH used (bp. 182 ° C.) as completely as possible is advantageous.
properties
2-Ethylhexylamine is a clear, colorless, pungent fish-like or ammonia-smelling liquid, which is highly corrosive and very irritating to the eyes and mucous membranes. At higher temperatures it forms flammable vapor-air mixtures. The compound has a flash point of 50 ° C. The explosion range is between 1.6% by volume (41 g / m 3 ) as the lower explosion limit (LEL) and 7.7% by volume (320 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 265 ° C. The substance therefore falls into temperature class T3.
Applications
2-Ethylhexylamine reacts with 2-chloronitrobenzene to form a yellow dye ( Automate Yellow 36 ), which is used to color fuels ( English fuel marker )
and its manufacture in 1998 at a Morton International Inc. site in Paterson (New Jersey) resulted in a subsequent, intensively investigated thermal runaway .
The tetrafluoroboric acid salt of 2-EHA is described as an additive in fuels to prevent carburetor icing at low temperatures.
Amine phosphate salts of 2-EHA with phosphoric acid esters of branched primary C 8 −C 16 alcohols are fuel additives for reducing deposits in internal combustion engines and are used as corrosion inhibitors for e.g. B. tin-plated aerosol cans are used. as well as salts of 2-EHA with N -acyl sarcosinates .
Together with corrosion inhibitors such as tolyltriazole , 2-EHA is used as an additive to reduce friction and wear in fuels and lubricants.
Sulphosuccinic acid amides with 2-EHA are versatile surface-active substances that are used as emulsifiers , dispersants , sizes for textiles, surfactants , flotation aids , etc.
When 2-EHA is reacted with ethylene carbonate , a urethane is produced that can be alkoxylated with ethylene oxide or propylene oxide to form low-foaming surfactants and wetting agents.
The substituted propionic acids formed in the reaction of 2-EHA with methyl 3-chlorosulfonylpropionate and subsequent hydrolysis are suitable as effective components in mixtures of corrosion inhibitors with antimicrobial properties.
According to a general instruction from Walter Reppe , the primary amine 2-ethylhexylamine also reacts with acrylic acid in a two- molar excess to form amphoteric octyliminodipropionate , which is widely used as a low-foaming and pH-stable hydrotrope (solubilizer) in industrial cleaners.
Depending on the pH value, octyliminodipropionate occurs as an electrically neutral zwitterion , in acid as a cationic ammonium ion and in alkaline as an anionic carboxylate ion.
2-Ethylhexylamine is used as a hardener in the crosslinking of bisphenol A diglycidyl ether .
2-EHA reacts with dodecenylsuccinic anhydride to form the corresponding cyclic imide ,
which, as an additive to coating systems for cathodic electrocoating (KTL), suppresses the formation of pinholes in the paint layer.
From 2-EHA and 5-norbornene-2,3-dicarboxylic acid anhydride , N- octyl-bicyclohepten-dicarboximide can be obtained , which acts as a synergist (pyrodone) for insecticides and as a repellent .
The most important pharmaceutical use of 2-ethylhexylamine is as a synthetic building block for the antiseptic hexetidine
The synthesis of hexetidine follows the general procedure of M. Senkus, whereby in a Mannich reaction between nitroethane and formaldehyde initially 2-methyl-2-nitropropane-1,3-diol is formed, which with two 2-EHA and one formaldehyde molecule Forms nitro precursor of hexetidine. The nitro compound is hydrogenated with hydrogen on Raney nickel to give hexetidine.
The hexetidine obtained in this synthesis route is only about 80% pure. The by-products can be removed by salt formation with naphthalene-1,5-disulfonic acid in hot alcohol-water mixtures, such as. B. isopropanol or methanol can be separated practically quantitatively.
Individual evidence
- ↑ 2-Ethyl-1-hexylamine data sheet from Sigma-Aldrich , accessed on January 25, 2016 ( PDF ).
- ↑ a b c d e f g h i j k l m Entry on 2-ethylhexylamine in the GESTIS substance database of the IFA , accessed on August 10, 2016(JavaScript required) .
- ↑ a b Oxea, safety data sheet, 2-ethylhexylamine
- ^ Carl L. Yaws: The Yaws Handbook of Physical Properties of Hydrocarbons and Chemicals . 2nd Edition. Elsevier Inc., Amsterdam 2015, ISBN 978-0-12-800834-8 , pp. 224 .
- ↑ MA Ayedi, Y. Le Bigot, H. Ammar, S. Abid, R. El Gharbi, M. Delmas: Synthesis of Primary Amines by One-Pot Reductive amination of aldehyde . In: Synth. Commun. tape 43 , no. 16 , 2013, p. 2127-2133 , doi : 10.1080 / 00397911.2012.714830 .
- ^ GV Sagar Reddy, GV Rao, RVK Subramanyam, DS Iyengar: A New Novel and Practical One Pot Methodology for Conversion of Alcohols to Amines . In: Synth. Commun. tape 30 , no. 12 , 2000, pp. 2233-2237 , doi : 10.1080 / 00397910008087402 .
- ↑ Chemistry Archive , One pot conversion of alcohols to amines , https://www.erowid.org/archive/rhodium/chemistry/alcohol2amine.html
- ↑ Patent US3022349 : Production of amines. Filed December 30, 1957 , published February 20, 1962 , Applicant: Union Carbide Corp., Inventor: RC Lemon, RC Myerly.
- ↑ US Chemical Safety and Hazard Investigation Board: Morton International Inc. Runaway Chemical Reaction ( Memento of May 12, 2016 in the Internet Archive )
- ↑ Patent US3118745 : Anti-stalling motor fuel. Filed January 18, 1961 , published January 21, 1964 , Applicant: Texaco Inc., Inventor: EC Knowles, EL Kay, KL Dille.
- ↑ Patent US3909214 : Multifunctional gasoline additive composition. Applied July 27, 1973 , published September 30, 1975 , applicant: EI Du Pont de Nemours and Company, inventor: P. Polss.
- ↑ Patent US4604226 : Aerosol corrosion inhibitors. Applied March 22, 1985 , published August 5, 1986 , applicant: EI Du Pont de Nemours and Company, inventor: PL Bartlett.
- ↑ Patent US5032317 : Process of inhibiting corrosion. Filed September 18, 1989 , published July 16, 1991 , applicant: EI Du Pont de Nemours and Company, inventor: PL Bartlett.
- ↑ Patent US5482521 : Friction modifiers and antiwear additives for fuels and lubricants. Applied May 18, 1994 , published January 9, 1996 , Applicant: Mobil Oil Corp., Inventors: NL Avery, EG Barry, JT Carey, LS Crocker, FW Feng, J. Hiebert, AG Horodysky, LA Nelson.
- ↑ Patent US2192906 : Diamides of aliphatic sulpho- and sulphato-dicarboxylic acids and processes of preparing them. Registered September 17, 1937 , published March 12, 1940 , Applicant: EI Du Pont de Nemours and Company, Inventor: WE Hanford, CO Henke.
- ↑ Patent US8629297B2 : Low-foaming surfactants. Registered on September 9, 2010 , published on January 14, 2014 , applicant: Cognis IP Management GmbH, inventor: C. Munzenberg, H. Wiethoff.
- ↑ Patent EP0015442A1 : Low-foam corrosion inhibitors with antimicrobial properties, which contain boric acid-alkanolamine reaction products as an effective principle. Registered on March 3, 1979 , published on September 17, 1980 , applicant: BASF AG, inventor: K. Oppenlaender, E. Getto, W. Kindscher, A. Hettche.
- ↑ Patent US2195974 : Process for producing new amino-carboxylic acids. Registered on July 10, 1937 , published on April 2, 1940 , applicant: IG Farbenindustrie AG, inventor: W. Reppe, H. Ufer.
- ↑ Akzo Nobel, Ampholak YJH-40, http://sc.akzonobel.com/en/fabric-cleaning/Pages/product-detail.aspx?prodID=8204
- ↑ ict, FlexisurfTM EHDP, http://ictchemicals.com/media/1306/flexisurf-ehdp-technical-data-sheet.pdf
- ↑ M. Tarnacka, M. Wikarek, S. Pawlus, K. Kaminski, M. Paluch: Impact of high pressure on the progress of polymerization of DGEBA cured with different amine hardeners: dielectric and DSC studies . In: RSC Adv. Band 5 , 2015, p. 105934-105942 , doi : 10.1039 / C5RA19766J .
- ↑ Patent US7153406B2 : Cathodic electrodeposition coating compositions and process for using same. Applied July 15, 2003 , published December 26, 2006 , Applicant: EI Du Pont de Nemours and Company, Inventors: H. Hoenig, E. Bambach, G. Pampoulidis, M. Valtrovic.
- ↑ Patent US2476512 : N-2-Ethyl hexyl-α, α'-di-keto-β, β '- (1,4-Δ 2 -cyclopentenylene) -pyrrolidine and synthesis thereof. Applied on March 8, 1947 , published July 19, 1949 , applicant: Van Dyke & Co., Inc., inventor: AA Schreiber.
- ^ A. Kleemann, J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances: Syntheses, Patents, Applications of the most relevant APIs . 5th edition. Thieme, Stuttgart 2009, ISBN 978-3-13-558405-8 .
- ↑ M. Senkus: Reaction of primary Aliphatic cardamines with formaldehyde and nitro paraffin . In: J. Am. Chem. Soc. tape 68 , no. 1 , 1946, p. 10–12 , doi : 10.1021 / ja01205a004 .
- ^ BM Vanderbilt, HB Haas: Aldehyde-nitroparaffin condensation . In: Ind. Eng. Chem. Band 32 , no. 1 , 1940, p. 34-38 , doi : 10.1021 / ie50361a007 .
- ↑ M. Senkus: The Preparation of Some Hexahydropyrimidines from Nitroparaffins1 . In: J. Am. Chem. Soc. tape 68 , no. 8 , 1946, pp. 1611-1613 , doi : 10.1021 / ja01212a073 .
- ↑ Patent US2387043 : 5- Aminohexahydropyrimidines and process for preparing same. Applied July 29, 1944 , published October 16, 1945 , Applicant: Commercial Solvents Corp., Inventor: M. Senkus.
- ↑ Patent US3749721 : Process for the production of pure hexetidine. Applied March 7, 1970 , published July 31, 1973 , Applicant: Warner-Lambert Pharmaceutical Co., Inventor: W. Herrmann, G. Satzinger.
Web links
- BASF: 2-ethylhexylamine
- Entry to 2-ethylhexylamine . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD













