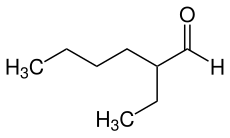
2-ethylhexanal
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-ethylhexanal | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 16 O | |||||||||||||||
| Brief description |
flammable, yellow liquid with a sharp, strong odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 128.21 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.82 g cm −3 (20 ° C) |
|||||||||||||||
| boiling point |
163 ° C |
|||||||||||||||
| Vapor pressure |
2.4 hPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.415 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-Ethylhexanal is a chemical compound from the group of aldehydes and is an isomer of octanal .
Extraction and presentation
2-Ethylhexanal can be obtained from butanal by a base-catalyzed aldol reaction and subsequent hydrogenation . More than 1000 tons were produced in 1988.
It can also be prepared directly from propylene by hydroformylation and subsequent aldol reaction in a one-pot reaction .
It can also be prepared from 2-ethylhexenal , which in turn is produced by condensation of butanal using aqueous sodium hydroxide solution .
properties
2-Ethylhexanal is a clear, flammable, yellow liquid with a sharp, strong odor. It is weakly acidic in water. The dynamic viscosity of the liquid is 0.9 mPa · s at 20 ° C.
use
In combination with other substances, 2-ethylhexanal is used as a surface disinfectant , solvent and as an intermediate product for the production of 2-ethylhexanol , 2-ethylhexanoic acid and 2-ethylhexylamine , as well as for the production of pharmaceuticals and fragrances. Condensation products of the compound are used as vulcanizing agents and antioxidants in the rubber industry.
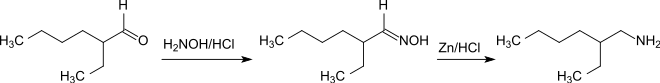
The amination of 2-ethylhexanal by reaction with hydroxylamine hydrochloride to form the oxime and subsequent reduction with zinc / hydrochloric acid is a simple one-pot method for the preparation of racemic 2-ethylhexylamine .
safety instructions
The vapors of 2-ethylhexanal can form an explosive mixture with air ( flash point 42 ° C).
Web links
- Entry for 2-ethylhexanal . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD
- Entry on 2-ethylhexanal in the Spectral Database for Organic Compounds (SDBS) of the National Institute of Advanced Industrial Science and Technology (AIST)
Individual evidence
- ↑ entry to ethylhexanal in CosIng database of the European Commission, accessed on 11 March 2020th
- ↑ a b c d e f g Entry on 2-ethylhexanal in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c d Toxicological assessment of 2-ethylhexanal (PDF) at the professional association raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
- ↑ 2-Ethylhexanal data sheet from Sigma-Aldrich , accessed on April 22, 2017 ( PDF ).
- ↑ Sumeet K Sharma, Raksh V Jasra: Synthesis of 2-Ethylhexanal from Propylene in a Single Pot Using an Ecofriendly Multifunctional Catalyst Synthesized by Intercalation of HRhCO (TPPTS) 3 Complex in the Interlayer Space of Hydrotalcite , Ind. Eng. Chem. Res. , 2011, 50 (5), pp. 2815-2821; doi: 10.1021 / ie1015365 .
- ↑ Process for the production of 2-ethylhexenal (patent-de).
- ↑ a b data sheet 2-ethylhexanal (PDF; 166 kB) at gischem.
- ↑ MA Ayedi, Y. Le Bigot, H. Ammar, S. Abid, R. El Gharbi, M. Delmas: Synthesis of Primary Amines by One-Pot Reductive amination of aldehyde . In: Synth. Commun. tape 43 , no. 16 , 2013, p. 2127-2133 , doi : 10.1080 / 00397911.2012.714830 .