2-ethylhexyl salicylate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| Simplified structural formula without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-ethylhexyl salicylate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 15 H 22 O 3 | |||||||||||||||
| Brief description |
clear colorless liquid with a faint floral odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 250.33 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.014 g cm −3 (25 ° C ) |
|||||||||||||||
| Melting point |
<−20 ° C |
|||||||||||||||
| boiling point | ||||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1,500–1,503 (20 ° C ) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Salicylic acid 2-ethylhexyl ester (2-ethylhexyl salicylate, octyl salicylate, octisalate) is an ester of salicylic acid with 2-ethylhexanol , which is used as a UV filter in sunscreens . The salicylic acid residue is responsible for UV absorption , the hydrophobic 2-ethylhexyl residue for water resistance in dermatological and cosmetic preparations. Octisalate is a weak UVB absorber with an absorption maximum at 305 nm and is mostly used in combination with other UV filters.
Isomers
There are two stereoisomeric forms of 2-ethylhexyl salicylate , ( S ) - and ( R ) -salicylic acid-2-ethylhexyl ester, which are mostly used as a 1: 1 mixture ( racemate ).
| Isomers of 2-ethylhexyl salicylate | ||
| Surname | ( S ) -Salicylic acid-2-ethylhexyl ester | ( R ) -Salicylic acid-2-ethylhexyl ester |
| other names | ( S ) -2-ethylhexyl salicylate | ( R ) -2-ethylhexyl salicylate |
| Structural formula |

|

|
presentation
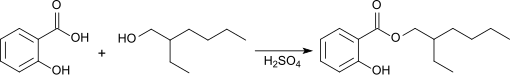
Salicylic acid-2-ethylhexyl ester is obtained as a racemate from the esterification of salicylic acid with ( RS ) -2-ethylhexanol in the presence of concentrated sulfuric acid .
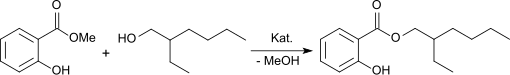
The transesterification of salicylic acid methyl ester with ( RS ) -2-ethylhexanol with basic catalysis, e.g. B. with sodium hydroxide or with tin (II) oxalate , dibutyltin dilaurate or tetraisopropyl orthotitanate gives racemic octyl salicylate in yields of over 90%.
properties
Salicylic acid-2-ethylhexyl ester is a clear, colorless liquid with a faint floral odor, which is insoluble in water and other polar solvents. Octyl salicylate is readily soluble in propylene carbonate , dipropylene glycol , tripropylene glycol and their ethers. Octyl salicylate is readily soluble in non-polar solvents and oils such as those used in the formulation of sunscreens. Other UV filters, such as B. Avobenzon , Enzacamen , Oxybenzone or Ethylhexyltriazon are dissolved by octyl salicylate.
In a Japanese in vitro study, octyl salicylate was found to have weak estrogenic activity. No reliable data are available on the reproductive and developmental toxicity of octyl salicylate. A group of Danish authors found a patient with a contact allergy due to the action of octyl salicylate.
Octyl salicylate is absorbed through the skin. It is excreted in the form of various metabolites, including the ester breakdown product salicylic acid and its secondary metabolite salicyluric acid, as well as several oxidized metabolites with an intact octyl salicylate skeleton.
use
With a specific extinction coefficient of approx. 180 at an absorption maximum of 305 nm, 2-ethylhexyl salicylate is a weak UVB absorber that is used in combination with other UV filters in sunscreens. With the conventional broad-spectrum (280-320 nm) -UVB filter octyl methoxycinnamate (octyl methoxycinnamate) results in synergistic effects with a significant increase of the Sun Protection Factor ( English SPF booster ). Octyl salicylate is used in sunscreens in concentrations of up to 5% (in Japan up to 10%).
Salicylic acid 2-ethylhexyl ester is approved as a UV filter in the USA and is sometimes used. a. manufactured by Ashland Inc. (under the brand name Escalol® 587), DSM (Parsol®EHS), Merck Millipore (Eusolex® OS) and Symrise (Neo Heliopan® OS).
Individual evidence
- ↑ Entry on ETHYLHEXYL SALICYLATE in the CosIng database of the EU Commission, accessed on December 29, 2019.
- ↑ a b c data sheet 2-ethylhexyl salicylate from Sigma-Aldrich , accessed on September 1, 2015 ( PDF ).
- ↑ Spectrum Laboratory Products, Inc., Octisalate ( PDF ).
- ↑ a b c Entry on 2-ethylhexyl salicylate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c Jeen, Jeescreen OS USP ( PDF ).
- ^ R. Rai, SC Shanmuga, CR Srinivas: Update on photoprotection . In: J. Indian Dermatol. tape 57 , no. 5 , 2012, p. 335-342 , doi : 10.4103 / 0019-5154.100472 .
- ↑ Patent US2347547 : Preparation method of isooctyl salicylate. Applied on August 15, 1941 , published April 25, 1944 , applicant: Sinclair Refining Co., inventor: WL Finley.
- ↑ Patent CN102775311 : Preparation method of isooctyl salicylate. Filed August 13, 2012 , published June 25, 2014 .
- ↑ Y.-K. Chen, Z.-H. Li, Y.-M. Huang: Synthesis of 2-ethylhexyl salicylate by transesterification with stannous oxalate as catalyst. ( Abstract ).
- ↑ Patent EP1652834 : Process for producing salicylate esters. Applied on October 25, 2005 , published on May 3, 2006 , applicant: Kao Corp., inventor: S. Ohno, T. Uehara, S. Kotachi, S. Tanaka.
- ^ LyondellBasell: Application Data, Solubility Screen, Ultraviolet Light Absorbers .
- ↑ K. Morohoshi, H. Yamamoto, R. Kanata, F. Shiraishi, T. Koda, M. Morita: Estrogenic activity of 37 components of commercial sunscreen lotions evaluated by in vitro assays . In: Toxicology in Vitro . tape 19 , 2005, pp. 457-469 , doi : 10.1016 / j.tiv.2005.01.004 .
- ↑ M. Axelstad, U. hatred, K. Lund chin Berg, P. Bjerregaard: Assessment of the endocrine disruptive potential of 23 UV filters (j.no. MST-656-00150) . Ed .: Danish Center of Endocrine Disrupters. February 2013 ( cend.dk [PDF]).
- ↑ CG Mortz, H. Thormann, A. Goossens, KE Andersen: Allergic contact dermatitis from ethylhexyl salicylate and other salicylates . In: dermatitis . tape 21 , no. 2 , 2010, p. E7-10 , doi : 10.2310 / 6620.2010.09090 .
- ↑ D. Bury, T. Brüning, HM Koch: Determination of metabolites of the UV filter 2-ethylhexyl salicylate in human urine by online SPE-LC-MS / MS . In: Journal of Chromatography B . tape 1110-1111 , 2019, pp. 59–66 , doi : 10.1016 / j.jchromb.2019.02.014 .
- ↑ D. Bury, P. Griem, T. Wildemann, T. Brüning, HM Koch: Urinary metabolites of the UV filter 2-Ethylhexyl salicylate as biomarkers of exposure in humans . In: Toxicology Letters . tape 309 , 2019, pp. 35-41 , doi : 10.1016 / j.toxlet.2019.04.001 .
- ↑ Patent WO2007042346 : Mixtures of ethylhexyl p-methoxycinnamate and ethylhexyl salicylate. Registered on August 28, 2006 , published on April 19, 2007 , applicant: Symrise GmbH & Co. KG, R. Langner, inventor: R. Langner.
- ^ BASF, Cross-Reference List of all UV filters used in the BASF Sunscreen Simulator [ PDF ( Memento from May 29, 2015 in the Internet Archive )].