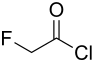
Fluoroacetyl chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Fluoroacetyl chloride | |||||||||||||||
| Molecular formula | C 2 H 2 ClFO | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 96.49 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.353 g cm −3 |
|||||||||||||||
| Melting point |
65-71 ° C |
|||||||||||||||
| solubility |
reacts with water |
|||||||||||||||
| Refractive index |
1.3831 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Fluoroacetyl chloride is a chemical compound from the group of carboxylic acid halides .
Extraction and presentation
Fluoroacetyl chloride can be obtained by reacting sodium fluoroacetate with phosphorus pentachloride .
properties
Fluoroacetyl chloride is a liquid that reacts with water.
use
Fluoroacetyl chloride is used to make other organic compounds.
Individual evidence
- ↑ a b c d Richard P. Pohanish: Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens . William Andrew, 2011, ISBN 1-4377-7869-0 , pp. 1338 ( limited preview in Google Book search).
- ↑ a b c chemical dictionary : Fluoroacetyl chloride
- ↑ Entry on fluoroacetyl chloride in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ^ William E. Truce: The Preparation of Fluoroacetyl Chloride. In: Journal of the American Chemical Society. 70, 1948, pp. 2828-2828, doi : 10.1021 / ja01188a524 .
- ↑ Entry on FLUOROACETYL CHLORIDE in the Hazardous Substances Data Bank , accessed on July 29, 2012.
