Functionality (chemistry)
| Monofunctional compounds |
|---|
 |
 |
|
|
| Difunctional compounds |
|---|
 |
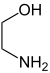 |
 |
| Trifunctional compounds |
|---|
 |
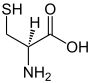 |
In chemistry, functionality describes the presence of functional groups in a molecule . In organic chemistry (and other areas of chemistry) the functionality of a molecule has a decisive influence on the reactivity of molecules. In polymer chemistry , the functionality of a monomer refers to the number of its polymerizable groups; it affects the formation and degree of crosslinking of polymers .
A monofunctional molecule has one function, a bifunctional molecule two, a trifunctional three functions, etc.
Functionality in Organic Chemistry and Materials Science
In organic chemistry, functionality is often used as a synonym for a functional group . So z. B. a hydroxyl group is also referred to as HO functionality.
As functionalization the introduction of functional groups is referred to, for example,
- the functionalization of a surface (e.g. silanization to specifically change the adhesion of this surface),
- the functionalization of nanoparticles of a metal or metal oxide to stabilize such nanoparticles or
- Replacing a CH bond with a functional group attached to the same carbon atom, known as CH functionalization ).
Functionality in Polymer Chemistry
According to IUPAC , the functionality of a monomer is defined as the number of bonds that a monomer or its repeating unit forms in a polymer with other monomers. With a functionality of f = 2, polymerization results in a linear polymer (a thermoplastic ). Monomers with a functionality f ≥ 3 lead to a branching point , which can lead to cross-linked polymers ( thermosets ). Monofunctional monomers therefore do not exist, since such molecules lead to chain termination .
From the average functionality of the monomers used, the reaching of the gel point can be calculated as a function of the reaction conversion . Side reactions can increase or decrease the functionality.
However, the IUPAC definition and the use of the literal meaning in organic chemistry differ in terms of the functionality of a double bond. If this has a functionality of two in polymer chemistry (since there are two points of attachment to the further polymer chain, one at each of the two adjacent carbon atoms), in organic chemistry a double bond is a functional group and therefore has a functionality of one.
Individual evidence
- ^ Kurt Peter C. Vollhardt, Neil Eric Schore: Organic Chemistry , p. 73 ( limited preview in the Google book search).
- ^ Riedel: Moderne Anorganische Chemie by Christoph Janiak, p. 401 ( limited preview in the Google book search).
- ↑ Alexander Langner, Anthony Panarello, Sandrine Rivillon, Oleksiy Vassylyev, Johannes G. Khinast, Yves J. Chabal: Controlled Silicon Surface Functionalization by Alkene Hydrosilylation , J. Am. Chem. Soc. , 2005, 127 (37), pp. 12798-12799 ( doi: 10.1021 / ja054634n ).
- ↑ Marie-Alexandra Neouze, Ulrich Schubert : Surface Modification and Functionalization of Metal and Metal Oxide Nanoparticles by Organic Ligands, Monatsh. Chem. 139 (2008), pp. 183-195.
- ↑ Dirk Steinborn: Fundamentals of organometallic complex catalysis , p. 305 ( limited preview in the Google book search
- ↑ a b entry on functionality, f of a monomer . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.FT07505 Version: 2.3.3.
- ↑ Koltzenburg: Polymers: Synthesis, Properties and Applications , p. 187 ( limited preview in the Google book search).
- ↑ Hans-Georg Elias: Macromolecules: Chemical Structure and Syntheses , p. 468 and 477 ( limited preview in the Google book search).
- ↑ Entry on chemical functionality . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.CT07503 Version: 2.3.3.