Gallopamil
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
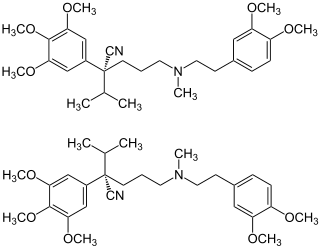
|
||||||||||||||||
| 1: 1 mixture of ( R ) -form (top) and ( S ) -form (bottom) | ||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Gallopamil | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula |
|
|||||||||||||||
| Brief description |
yellow viscous oil (gallopamil) |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | ||||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
240–245 ° C (0–26.6 Pa, gallopamil) |
|||||||||||||||
| Refractive index |
1.5402 (hydrochloride) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Gallopamil is a drug from the nitrile group . It exists as a racemate .
properties
It is a calcium antagonist and calcium channel blocker . This property has the consequence that the Ca 2+ ions cannot diffuse into the cells as in the undisturbed state . A muscle contraction remains of why or relaxes, causing the blood vessels wide or the heart muscle itself is relaxed.
use
Gallopamil is used in medicine for the treatment of cardiovascular diseases , especially for diseases of the coronary arteries , e.g. B. angina pectoris , cardiac arrhythmias or high blood pressure are used.
pharmacy
Trade names
Monopreparate
Procorum (D; former trade name, wastaken off the marketin April 2012 by the manufacturer Abbot in Germany)
literature
- Entry on gallopamil. In: Römpp Online . Georg Thieme Verlag, accessed on September 17, 2011.
Individual evidence
- ↑ a b c d e f g Entry on Gallopamil. In: Römpp Online . Georg Thieme Verlag, accessed on September 17, 2011.
- ↑ Data sheet (±) -Methoxyverapamil hydrochloride, ≥98% from Sigma-Aldrich , accessed on February 15, 2013 ( PDF ).
- ↑ Gross AS, Eichelbaum M, Mörike K, Mikus G: Pharmacokinetics and pharmacodynamics of R- and S-gallopamil during multiple dosing . In: British Journal of Clinical Pharmacology . 49, No. 2, February 2000, pp. 132-138. PMID 10671907 . PMC 2014898 (free full text).