Geneticin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
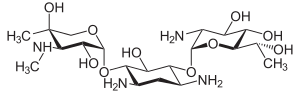
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Geneticin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 20 H 40 N 4 O 10 | |||||||||||||||
| Brief description |
colorless powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 496.55 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Geneticin ( G-418 ) is an antibacterially active substance ( antibiotic ) from the group of aminoglycosides . It mediates its antibiotic effect by inhibiting protein synthesis . Since geneticin also inhibits protein synthesis in cells of higher organisms (e.g. mammalian cells) to a particular extent, geneticin is not used therapeutically. Its most important field of application is cell biology , in which geneticin is used to select genetically modified cell lines .
Occurrence
Geneticin was first isolated in 1974 from the Micromonospora rhodorangea strain NRRL 5326.
Mechanism of action
Like the other aminoglycosides, geneticin inhibits cellular protein synthesis. While many aminoglycosides exclusively inhibit prokaryotic 70S ribosomes in various stages of translation, geneticin also has an effect on protein synthesis in eukaryotic cells. Geneticin binds to the 80S ribosomes from fungi, algae, plant and animal cells. Geneticin bound to ribosomes prevents translation elongation .
application areas
Geneticin is used in particular in cell biology for the selection of stably transfected eukaryotic cells. On the one hand, the cell toxicity of geneticin for eukaryotic cells and, on the other hand, the possibility of intracellular geneticin inactivation by introducing a neomycin-geneticin resistance gene (Neo r ) is exploited. The genes responsible for resistance to geneticin code for aminoglycoside phosphotransferases (APT). These phosphorylate geneticin on the hydroxyl groups and thus suppress the effect of the substance.
Individual evidence
- ↑ a b c data sheet G 418 disulfate salt from Sigma-Aldrich , accessed on January 27, 2017 ( PDF ).
- ↑ Data sheet from Calbiochem ( Memento from September 28, 2007 in the Internet Archive ) (PDF; 25 kB).
- ↑ Wagman, GH et al. (1974): Antibiotic G-418, a new Micromonospora-produced aminoglycoside with activity against protozoa and helminths: fermentation, isolation, and preliminary characterization. In: Antimicrob. Agents Chemother. Vol. 6, pp. 144-149. PMID 15828184
- ↑ Bar-Nun, S. et al. (1983): G-418, an elongation inhibitor of 80 S ribosomes. In: Biochim. Biophys. Acta . Vol. 741, pp. 123-127. PMID 6193810 ; doi: 10.1016 / 0167-4781 (83) 90018-0
- ↑ a b Davies, J. and Jimenez, A. (1980): A new selective agent for eukaryotic cloning vectors . In: Am J Trop Med Hyg . 29 (5 Suppl); 1089-1092; PMID 7001938 ; doi : 10.4269 / ajtmh.1980.29.1089 .
- ^ Spectrum Akademischer Verlag GmbH: Herder LEXICON of biochemistry and molecular biology spectrum . Heidelberg-Berlin-Oxford 1995, ISBN 3-86025-158-9 (Vol. 2).
- ↑ Hadfield, C. et al. (1990): G418-resistance as a dominant marker and reporter for gene expression in Saccharomyces cerevisiae. In: Curr. Genet. Vol. 18, pp. 303-313, PMID 2174744 .
