Hafnium (III) iodide
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
|
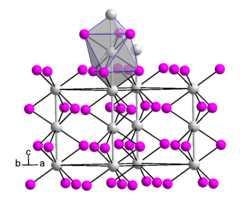
__ Hf 3+ __ I - crystal structure of stoichiometrically composed hafnium (III) iodide |
|||||||
| General | |||||||
| Surname | Hafnium (III) iodide | ||||||
| other names |
Hafnium triiodide |
||||||
| Ratio formula | HfI 3 | ||||||
| Brief description |
black solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 559.2 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| density |
2.74 g cm −3 (HfI 3.5 ) |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Hafnium (III) iodide is a chemical compound of hafnium from the group of iodides .
Extraction and presentation
Hafnium (III) iodide can be obtained by reducing hafnium (IV) iodide, for example with aluminum. The resulting products have a composition between HfI 3.00 to HfI 3.50 (Hf 0.86 I 3 ).
properties
Hafnium (III) iodide is a black solid. It has a crystal structure of the zirconium (III) iodide type. While the structure of HfI 3 can still be described in the space group P 6 3 / mcm (space group no. 193) with equidistant Hf-Hf distances, this is no longer possible for Hf 0.86 I 3 . Due to the statistical understaffing of the Hf layers, trimers are formed within the octahedron chain. In the space group R 3 m (space group no. 166) is possible this arrangement. The compound decomposes at temperatures above 275 ° C.
Individual evidence
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, 2016, ISBN 978-1-4987-5429-3 , pp. 105 ( limited preview in Google Book search).
- ↑ a b Jan Arndt Beekhuizen: New studies on halides of titanium and hafnium , dissertation, Cologne 2006
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b R. JH Clark, DC Bradley, P. Thornton: The Chemistry of Titanium, Zirconium and Hafnium Pergamon Texts in Inorganic Chemistry . Elsevier, 2013, ISBN 978-1-4831-5921-8 , pp. 432 ( limited preview in Google Book search).
- ^ HJ Emeléus, AG Sharpe: Advances in Inorganic Chemistry and Radiochemistry . Academic Press, 1971, ISBN 978-0-08-057862-0 , pp. 93 ( limited preview in Google Book search).
- ^ Christoph Janiak, Hans-Jürgen Meyer, Dietrich Gudat, Ralf Alsfasser: Riedel Modern Inorganic Chemistry . Walter de Gruyter, 2012, ISBN 978-3-11-024901-9 , p. 357 ( limited preview in Google Book search).
- ↑ Jan Beekhuizen, Anja-Verena Mudring, Gerd Meyer: Linear Trimeric Hafnium Clusters in volume 0.86 (1) I3. In: Crystals. 1, 2011, p. 40, doi : 10.3390 / cryst1020040 .