Ivanov reaction
The Ivanov reaction , also Ivanov reaction, is a name reaction in organic chemistry and named after the Bulgarian chemist Dimitr Ivanov (1894–1975). He first described this reaction in 1931.
Overview reaction
In the Ivanov reaction, a Grignard compound binds to an electrophile in the α position. Aryl and phenyl groups can function as radical (R) . Furthermore, aldehydes and ketones are mainly used as electrophiles ( E ).
General
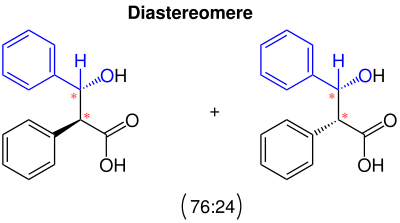
The reaction has yields of 60% to 91%, depending on the process. Two diastereomers are formed which could be separated by chromatography on a stationary phase of silica gel . It was found that 76% of one diastereomer with a melting point of 178 ° C and the other only 24% with a melting point of 143 ° C could be obtained.
Reaction mechanism
First, a Di is anion or the so-called Ivanov reagent from aryl - acetic acid produced. An electrophilic reaction with the Grignard compound takes place ( 1 ). This creates another carbonyl group , which in turn also reacts electrophilically with a Grignard compound ( 2 ). The result is the Ivanov reagent, which has more of an ionic character in terms of its bond with the magnesium (dianion).
The Ivanov reagent reacts with electrophiles , preferably carbonyl compounds ( aldehydes , ketones ), isocyanates or alkyl halides . In the following example, the Ivanov reagent is made from phenylacetic acid and two equivalents of a Grignard compound . Benzaldehyde ( marked in blue ) is used as the electrophile . In 3 , an electrophilic attack by the aldehyde on the double bond of the Ivanov reagent takes place, whereby the magnesium binds to the oxygen of the benzaldehyde by flipping the electrons ( 4 ). The end product 5 is obtained by hydrolysis .
Stereoselectivity
The Ivanov reaction produces two diastereomers . These arise in a ratio of 76:24. The reason for this is the steric hindrance of the phenyl group (s). This steric hindrance of the Ivanov reaction was described by Zimmermann and Traxler via a Zimmermann-Traxler transition state named after them . Toullec et al. studied the kinetics of the reaction. The stereocenters are marked with red asterisks in the figure.
See also
literature
- Bradford P. Mundy, Michael G Ellerd, Frank G. Favaloro Jr .: Name Reactions and Reagents in Organic Syntheses, second Edition, Wiley-Interscience, 2005, p. 342, ISBN 0-471-22854-0 .
- The Merck Index, 9th Edition, Merck & Co. 1976, ONR- (to be filled in), ISBN 0911910-26-3
- Reaction mechanisms, Reinhard Brückner, 3rd edition, Spectrum Akademischer Verlag, 2004, pp. 556–559
Individual evidence
- ^ Bradford P. Mundy, Michael G Ellerd, Frank G. Favaloro Jr .: Name Reactions and Reagents in Organic Syntheses , 2nd Edition, Wiley-Interscience, 2005, p. 342, ISBN 0-471-22854-0 .
- ^ Howard E. Zimmerman, Marjorie D. Traxler: Journal of the American Chemical Society . tape 79 , 1957, pp. 1920–1923 , doi : 10.1021 / ja01565a041 .
-
↑ D. Ivanov, A. Spassoff: Bulletin de la Société Chimique de France, 4e sér. tape 49 , 1931, pp. 19 . D. Ivanov, A. Spassoff: Bulletin de la Société chimique de France, 4e sér. tape
49 , 1931, pp. 375 . -
↑ D. Ivanov et al. : Bulletin de la Société chimique de France, 4e sér. tape 51 , 1932, p. 1321 . D. Ivanov et al. : Bulletin de la Société chimique de France, 4e sér. tape
51 , 1932, p. 1325 . D. Ivanov et al. : Bulletin de la Société chimique de France, 4e sér. tape
51 , 1932, p. 1331 . - ↑ B. Blagoev, D. Ivanov: Syntheses with Polyfunctional Organomagnesium Compounds . In: Synthesis . tape 1970 , no. 12 , 1970, pp. 615-627 , doi : 10.1055 / s-1970-21652 .
- ↑ D. Ivanov, G. Vassilev, I. Panayotov: Syntheses and Reactions of Organolithium Reagents Derived from Weakly Acidic CH-Compounds . In: Synthesis . tape 1975 , no. 2 , 1975, p. 83-98 , doi : 10.1055 / s-1975-23669 .
- ↑ CR Hauser, WR Dunnavant: α, β-Diphenylpropionic Acid In: Organic Syntheses . 40, 1960, p. 38, doi : 10.15227 / orgsyn.040.0038 ; Coll. Vol. 5, 1973, p. 526 ( PDF ).
- ^ Jean Toullec, Margarita Mladenova, Francoise Gaudemar-Bardone, Balgoi Blagoev: Kinetics and mechanism of the Ivanov reaction: reaction of aldehydes and ketones with phenylacetic acid magnesium enediolate . In: The Journal of Organic Chemistry . tape 50 , no. 14 , July 1, 1985, pp. 2563-2569 , doi : 10.1021 / jo00214a030 .
- ^ Reaction mechanisms, Reinhard Brückner, 3rd edition, Spektrum Akademischer Verlag, 2004, p. 556.