Potassium tetraperoxochromate
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
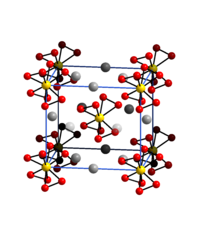
|
|||||||
| __ Cr 5+ __ K + __ O - | |||||||
| Crystal system | |||||||
| Space group |
I 4 2 m (No. 121) |
||||||
| Lattice parameters |
a = 670.3 pm |
||||||
| General | |||||||
| Surname | Potassium tetraperoxochromate | ||||||
| other names |
Potassium tetraperoxochromate (V) |
||||||
| Ratio formula | K 3 CrO 8 | ||||||
| Brief description |
red-brown solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 297.3 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Potassium tetraperoxochromate is a chemical compound from the group of chromates .
Extraction and presentation
Potassium tetraperoxochromate can be obtained by reacting potassium hydroxide , chromium (VI) oxide and hydrogen peroxide .
It can also be made by reacting potassium chromate with potassium hydroxide and hydrogen peroxide.
properties
Potassium tetraperoxochromate forms deep red-brown octahedral crystals that are moderately soluble in cold water and insoluble in alcohol and ether and explode when heated to 170 ° C. The salt can be kept for a long time without decomposition, but it can explode spontaneously. The crystal structure ( tetragonal , space group I 4 2 m (space group no. 121) ) contains tetraperoxochromate (V) ions, in which each chromium atom is coordinated by eight oxygen atoms.
use
Potassium tetraperoxochromate (V) can be used to generate singlet oxygen . It has also been used to induce arthritis in mice.
Individual evidence
- ^ R. Stomberg: Least-squares refinement of the crystal structure of potassium peroxochromate . In: Acta Chemica Scandinavica , 1963 , 17 , pp. 1563-1566 doi : 10.3891 / acta.chem.scand.17-1563 .
- ↑ a b c d Georg Brauer: Handbook of preparative inorganic chemistry . 3., reworked. Edition. tape III . Enke, Stuttgart 1981, ISBN 3-432-87823-0 , pp. 1528 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ J. Derek Woollins: Inorganic Experiments . John Wiley & Sons, 2010, ISBN 978-3-527-32472-9 , pp. 50 ( limited preview in Google Book search).
- ↑ Jander-Blasius, Textbook of Analytical and Preparative Inorganic Chemistry, 8th Edition, S. Hirzel Verlag Stuttgart, 1969
- ↑ R. M. Wood, K. A. Abboud, R. & C. Palenik, G. J. Palenik: Bond valence sums in coordination chemistry. Calculation of the oxidation state of chromium in complexes containing only Cr-O bonds and a redetermination of the crystal structure of potassium tetra (peroxo) chromate (V) . In: Inorganic Chemistry , 2000 , 39 , pp. 2065-2068 ( doi : 10.1021 / ic990982c ).
- ↑ Entry on potassium chromate. In: Römpp Online . Georg Thieme Verlag, accessed on July 10, 2018.
- ↑ EK Hodgson, I. Fridovich: Production of superoxide radical during the decomposition of potassium peroxochromate (V). In: Biochemistry. 13, 2002, p. 3811, doi : 10.1021 / bi00715a030 .
- ↑ Ralf Miesel, Hans Kröger, Maciej Kurpisz, Ulrich Weser: Induction of Arthritis in Mice and Rats by Potassium Peroxochromate and Assessment of Disease Activity by Whole Blood Chemiluminescence and pertechnetate-Imaging. In: Free Radical Research. 23, 2009, p. 213, doi : 10.3109 / 10715769509064035 .

