Lateral escapement
As lateral inhibition ( environment inhibition, lateral inhibition, or Lateralhemmung Lateralinhibition ) is referred to in the neurobiology a Verschaltungsprinzip of nerve cells by an active nerve cell inhibits the activity of the neighboring cells. This general neurophysiological principle occurs throughout the central nervous system - the best researched example is the excitation of the cones by daylight, which inhibits the transmission of stimuli by the rods .
Interconnection principle of the lateral inhibition
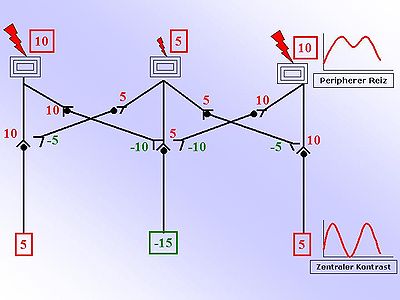
The drawing on the right makes this principle easier to understand. You can see three receptors, for example on the surface of the skin. The two outer receptors are more strongly excited than the inner receptor. The numbers opposite are any relative units that should provide information about the strength. The larger the number, the greater the excitement. Positive, red numbers are exciting, negative, green numbers are inhibiting. The strength of the excitation is now converted into an action potential frequency and passed on. The circuit diagram shown shows inhibitory interneurons that convert an exciting incoming signal ( afference ) into an inhibitory (inhibitory) continuing signal. The switch from excitation to inhibition occurs by means of different neurotransmitters (inhibitory: GABA , glycine , adrenaline ). These inhibitory interneurons weaken the adjacent transmission to a so-called second neuron. This weakening occurs proportionally to the excitation of the interneuron (inhibition: negative, green numbers in the figure). By simply adding up the exciting and inhibiting influences on the second neuron, a contrast enhancement results. This is shown graphically in the diagram opposite.
Occurrence
It occurs in intricately intertwined circuits, such as those located in ganglion cell nodes or, in the eye, in the upper layers of the retina .
In the retina, the inhibitory interneurons are called horizontal cells and serve to connect the photoreceptors (rods and cones) to the sides . However , the retina has other, complex possibilities for interconnection to increase the image contrast and the rapid detection of movements. The result of the lateral inhibition is above all the contrast enhancement and thus the formation of shape boundaries, which can serve as the basis for spatial orientation .
Lateral inhibition in developmental biology
The term lateral inhibition is also used to describe a regulatory path in developmental biology. It is conveyed , for example, through the notch signal path . Since the development of multicellular organisms is a complex process, precise control of proliferation and specialization is required. It is assumed here that lateral inhibition via the Notch signal path plays an important role. With lateral inhibition, a binary decision is usually made about the further development of a cell.
In this case, all cells are initially of the same "type" until a cell has an asymmetry in relation to delta due to a stochastic process and expresses more delta than all surrounding cells. This asymmetry is reinforced by a subsequent positive feedback and in the other cells Delta is inhaled and more Notch is expressed. So these cells become cells of the other "type".
This process plays a role, for example, in the formation of an exact structure of hair cells and supporting cells in the ear. It was shown in chickens that Notch first initiates the formation of so-called prosensory cells and then the amount and distribution of the final hair cells is regulated by lateral inhibition.
Lateral inhibition as inspiration for artificial neural networks (machine learning)
In so-called " convolutional neural networks " (CNNs) the process of lateral inhibition is simulated by selecting neurons for the most active neurons in a max pooling step within a layer, while neighboring more inactive neurons are switched off for further calculation steps of the network.
See also
Web links
Individual evidence
- ↑ Ashish Bakshi, Kuntal Ghosh: A Neural Model of Attention and Feedback for Computing Perceived Brightness in Vision . In: Handbook of Neural Computation . Elsevier, 2017, ISBN 978-0-12-811318-9 , pp. 487-513 , doi : 10.1016 / b978-0-12-811318-9.00026-0 ( elsevier.com [accessed October 23, 2019]).
- ^ Friedrich Zettler, Matti Järvilehto: Lateral inhibition in an insect eye . In: Journal for Comparative Physiology . tape 76 , no. 3 , 1972, ISSN 0340-7594 , p. 233–244 , doi : 10.1007 / BF00303230 ( springer.com [accessed October 25, 2019]).
- ^ Richard H. Kramer, Christopher M. Davenport: Lateral Inhibition in the Vertebrate Retina: The Case of the Missing Neurotransmitter . In: PLOS Biology . tape 13 , no. 12 , December 10, 2015, ISSN 1545-7885 , p. e1002322 , doi : 10.1371 / journal.pbio.1002322 , PMID 26656622 , PMC 4675548 (free full text) - ( plos.org [accessed October 23, 2019]).
- ↑ Cameron HG Wright, Steven F. Barrett: Biomimetic Vision Sensors . In: Engineered Biomimicry . Elsevier, 2013, ISBN 978-0-12-415995-2 , pp. 1–36 , doi : 10.1016 / b978-0-12-415995-2.00001-5 ( elsevier.com [accessed October 23, 2019]).
- ↑ Webvision: The Organization of the retina and visual system . University of Utah Health Sciences Center, Salt Lake City (UT) 1995, chap. "Simple Anatomy of the Retina", PMID 21413389 ( nih.gov [accessed October 23, 2019]).
- ↑ Bruce Appel, Lee Anne Givan, Judith S Eisen: [No title found] . In: BMC Developmental Biology . tape 1 , no. 1 , 2001, p. 13 , doi : 10.1186 / 1471-213X-1-13 , PMID 11495630 , PMC 37243 (free full text) - ( biomedcentral.com [accessed October 24, 2019]).
- ↑ Makoto Sato, Tetsuo Yasugi, Yoshiaki Minami, Takashi Miura, Masaharu Nagayama: Notch-mediated lateral inhibition regulates proneural wave propagation when combined with EGF-mediated reaction diffusion . In: Proceedings of the National Academy of Sciences . tape 113 , no. 35 , August 30, 2016, ISSN 0027-8424 , p. E5153 – E5162 , doi : 10.1073 / pnas.1602739113 , PMID 27535937 , PMC 5024646 (free full text) - ( pnas.org [accessed October 24, 2019]).
- ↑ N. Perrimon, C. Pitsouli, B.-Z. Shilo: Signaling Mechanisms Controlling Cell Fate and Embryonic Patterning . In: Cold Spring Harbor Perspectives in Biology . tape 4 , no. 8 , August 1, 2012, ISSN 1943-0264 , p. a005975 – a005975 , doi : 10.1101 / cshperspect.a005975 , PMID 22855721 , PMC 3405863 (free full text) - ( cshlp.org [accessed October 24, 2019]).
- ↑ N. Daudet: Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation . In: Development . tape 132 , no. 3 , January 5, 2005, ISSN 0950-1991 , p. 541–551 , doi : 10.1242 / dev.01589 ( biologists.org [accessed October 25, 2019]).
- ↑ Cao, C., Huang, Y., Wang, Z., Wang, L., Xu, N., & Tan, T .: Lateral inhibition-inspired convolutional neural network for visual attention and saliency detection . In: Thirty-Second AAAI Conference on Artificial Intelligence . April 2018.
- ↑ Bruno Jose Torres Fernandes, George DC Cavalcanti, Tsang Ing Ren: Lateral Inhibition Pyramidal Neural Network for Image Classification . In: IEEE Transactions on Cybernetics . tape 43 , no. 6 , December 2013, ISSN 2168-2267 , p. 2082-2092 , doi : 10.1109 / TCYB.2013.2240295 ( ieee.org [accessed November 1, 2019]).