Light disk microscopy

The light sheet fluorescence microscopy or light disc fluorescence microscopy ( LSFM from English Light Sheet Fluorescence Microscopy , also SPIM from English single plane illumination microscopy , Selective plane illumination microscopy , also Light Sheet Microscopy and light sheet microscopy ) is a fluorescence microscopic method, illuminated in which only a thin layer in the sample typically a few microns . Compared with conventional fluorescence microscopy, this leads to better resolution and a significantly reduced image background. It also reduces the negative effects of bleaching or light-induced stress in biological samples.

The method is used in cell biology and also for fluorescence studies on living organisms. Many applications can also be found in long-term observations of embryonic development in model organisms ( developmental biology ).
Light disk microscopy, developed at the beginning of the 21st century, introduced an illumination geometry into fluorescence microscopy that was already used successfully in darkfield microscopy in a comparable form at the beginning of the 20th century with the slit ultrasonic microscope .
construction
Basic structure
In this type of microscopy, excitation light is radiated perpendicular to the direction of observation (typically by a laser that is matched to the absorption bands of the selected fluorescent dye , e.g. from an argon laser at 488 nm for green fluorescent protein ). The expanded, collimated laser beam is only focused in one direction with the help of a cylindrical lens. The result is a “light disk” in the focus that only illuminates a thin layer within the sample. In order to increase the numerical aperture of the lens (and thus to reduce its thickness), a combination of a cylinder lens and a microscope objective is usually used. Fluorescent dye molecules in the illuminated layer are excited to fluoresce, which is then observed perpendicular to it with the aid of a light microscope. In order to have enough space for the projection of the lens, so-called immersion lenses with a large working distance (e.g. 2-3 mm with a numerical aperture of 1) are usually used, which are completely immersed in water or in a buffer solution. Therefore, in most SPI microscopes, a water-filled sample chamber is constructed around the sample, which also allows the sample to be examined under physiological conditions (e.g. physiological salt concentrations and 37 ° C).
The focusing of different parts of the sample takes place here (in contrast to wide-field fluorescence microscopy ) typically not by moving the objective (then the position of the light disc would have to be changed accordingly), but by moving the sample itself.
Some technical enhancements
Since the first implementations of the SPIM principle, some extensions have been introduced that improve the properties of an SPI microscope or simplify the structure:
- Two opposing light discs reduce typical SPIM artifacts, such as B. Shadows (see first z-stack above).
- In addition to the opposing light panes, it was proposed in 2012 to integrate two detection arms into a SPIM, which significantly speeds up the measurement of z and rotation stacks. Both together are necessary for a complete 3D reconstruction of the sample.
- The light disk can also be created by scanning a normal laser focus up and down. This method also makes it possible to use self-sustaining laser beams such as Bessel beams , which significantly increase the depth of penetration of the lightsheet into the sample, because the negative effect of scattering on the sample is reduced.
- In the so-called Oblique Plane Microscopy (OPM), the detection lens is also used to project the lightsheet. This leaves the objective at an angle of about 60 ° and additional optics in the detection beam path of the microscope are used to tilt the focal plane or detection plane accordingly.
- A fluorescence excitation according to the two-photon principle (two photons of double wavelength excite the fluorophore together) was realized. Above all, this illumination modality improves the penetration depth in scattering samples.
- SPIM was used as a microscopy technique in conjunction with fluorescence correlation spectroscopy (SPIM-FCS) to measure spatially resolved mobility maps of fluorescent particles (e.g. fluorescent microspheres, quantum dots or fluorescence -labeled proteins) in living biological samples / organisms.
- LSFM has also been combined with super-resolution microscopy techniques to overcome Abbe's limit of resolution. The stimulated emission depletion principle (STED) was also implemented for the lens illumination in order to reduce the thickness of the lens and thus improve the longitudinal resolution.
Sample holder
The separation of the illumination and detection beam paths in most LSFMs and the fact that these are mostly arranged in a horizontal plane make special sample holders necessary. The samples are often suspended from above or mounted on a standing holder (see images on the right). Different holders have been developed for different samples:
- Dead (e.g. fixed ) and large samples can be placed on a holder in the sample chamber e.g. B. be attached with adhesive.
- Larger living organisms (embryos ...) can be sedated and then enclosed in a soft gel cylinder that is pushed out of a glass or plastic capillary
- Adherent cells are allowed to grow directly on small glass plates, which then hang from above in the sample chamber.
- Plants can grow in clear gels if the gels are made with a suitable growth and nutrient medium. The gels are typically removed from around the observation region so that they do not degrade the quality of the light disk through scattering and absorption.
- Liquid samples (e.g. for SPIM-FCS) can be shrink-wrapped in small packs made from a thin plastic film. It is important that the plastic film has the same refractive index as the surrounding medium in order not to interfere with the imaging performance of the SPIM.
Some LSFMs have also been developed that implement the excitation and detection beam path in an upright plane. This means that samples can also be assembled using standard microscopic methods (e.g. cells in a Petri dish). It is also possible to combine an LSFM with an inverted microscope below.
Resolving power
With SPIM, observation takes place via a microscope objective, which is immersed in the water-filled sample chamber and images the sample directly. The lateral resolution is thus completely given by this objective and reaches a maximum of about half a wavelength to a wavelength (ie, for example, with green fluorescence about 250-500 nm). The axial resolution is significantly worse (typically by more than a factor of 4). It can, however, be improved somewhat by making the light sheet thinner so that fluorescence is only excited in part of the observation focus. Ideally, the axial resolution is the same as the lateral one.
In comparison with a normal wide field microscope, the axial resolution is significantly better. For small numerical apertures, the axial resolution is even better than for confocal microscopes; for larger numerical apertures it is still of a comparable order of magnitude. Compared to confocal microscopy, the image is not scanned in 3D, but in slices, from which all image points can be recorded simultaneously.
history
At the beginning of the 20th century, RA Zsigmondy introduced the ultramicroscope, a new lighting method into dark field microscopy. Sunlight or a white light lamp illuminates an optical gap , which is then imaged into the sample with a lens. Small particles passing through the light sheet formed in this way can be observed by means of their scattered light at a right angle to the illumination with an observation microscope. This microscope allowed the observation of particles smaller than the optical resolution of the observation microscope and led to the award of the Nobel Prize to Zsigmondy in 1925.
The first application of this lighting principle for fluorescence microscopy was from 1993 by Voie et al. published under the name Orthogonal-plane fluorescence optical sectioning (OPFOS). At that time to map the internal structure of the cochlea with a resolution of 10 µm laterally and 26 µm longitudinally, but with a sample size in the millimeter range. A simple cylindrical lens was used to shape the lens. The process was further developed and improved from 2004 onwards. After that, the technology was widely used and is still being adapted today with new variants (see above). Ultramicroscopes with fluorescence excitation and low resolution have been commercially available since 2010, and SPIM microscopes since 2012. A good overview of the development can be found e.g. B. in Ref. In 2012/2013 the first open source projects on LSFMs were started. They publish the complete construction plan, including the necessary software for setting up an LSFM.
application
SPIM is often used in developmental biology, where e.g. B. enables long-term observation of embryonic development. It can, however, also be combined with techniques such as fluorescence correlation spectroscopy in order to enable spatially resolved mobility measurements of fluorescent particles (e.g. beads, quantum dots, fluorescence-labeled proteins) in (biological) samples.
SPIM image of a living cell spheroid whose cell nuclei are labeled with the fusion protein H2B -HcRed.
SPIM images of an amoeba whose membrane was labeled with DiI
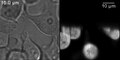
HeLa cells expressing tetramers of the green fluorescent protein EGFP . A transmitted light image can be seen on the left and an image recorded with a SPIM on the right. Typical SPIM artifacts such as shadowing can be clearly seen. The light came in from below.
Volmetric reconstruction of the SPIM z-stack on the left
Brownian motion of fluorescent latex beads (diameter approx. 20 nm) in water, recorded with an SPI microscope.
literature
- Review article:
- J. Huisken, DYR Stainier: Selective plane illumination microscopy techniques in developmental biology . In: Development . 136, No. 12, May 22, 2009, pp. 1963-1975. doi : 10.1242 / dev.022426 .
- PA Santi: Light Sheet Fluorescence Microscopy: A Review . In: Journal of Histochemistry & Cytochemistry . 59, No. 2, February 1, 2011, pp. 129-138. doi : 10.1369 / 0022155410394857 .
Web links
- Video of a typical experiment from developmental biology with a SPIM : In the linked video, the development of a fruit fly embryo was observed for about 20 hours. It shows two projections of the complete 3D data set.
- OpenSPIM : OpenSPIM is a platform to build, adapt and improve SPIM technology.
- Microscopy: nerve tracts of a chicken embryo (light disk microscopy)
Individual evidence
- ↑ Philipp J. Keller, Ernst HK Stelzer: Light disk microscopy in molecular cell biophysics In: LABORWELT. 7th year, no. 5, 2006, pp. 18-21 ( online version ( Memento of the original from January 20, 2013 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this note .; PDF; 7.5 MB).
- ↑ U. Krzic, S. Günther, L. Hufnagel, D. von Gegerfelt, H. Karlsson, E. Illy, J. Hel: Light disk fluorescence microscopy (SPIM) and laser excitation in orange for imaging living organisms. In: BioPhotonics. No. 1, 2011, pp. 42-44 ( online version ).
- ↑ a b A. H. Voie, DH Burns, FA Spelman: Orthogonal-plane fluorescence optical sectioning: Three-dimensional imaging of macroscopic biological specimens . In: Journal of Microscopy . 170, No. 3, June 1993, pp. 229-236. doi : 10.1111 / j.1365-2818.1993.tb03346.x .
- ↑ a b c J. Huisken, J. Swoger, F. Del Bene, J. Wittbrodt, EH Stelzer: Optical sectioning deep inside live embryos by selective plane illumination microscopy. In: Science . Volume 305, No. 5686, 2004, pp. 1007-1009, doi: 10.1126 / science.1100035 , PMID 15310904 .
- ↑ Timo Mappes, Norbert Jahr, Andrea Csaki, Nadine Vogler, Juergen Popp, Wolfgang Fritzsche: The Invention of Immersion Ultramicroscopy in 1912-The Birth of Nanotechnology? . In: Angewandte Chemie International Edition . 51, No. 45, November 5, 2012, pp. 11208-11212. doi : 10.1002 / anie.201204688 .
- ↑ a b c K. Greger, J. Swoger, EH Stelzer: Basic building units and properties of a fluorescence single plane illumination microscope. In: The Review of scientific instruments. Volume 78, No. 2, 2007, p. 023705, PMID 17578115 .
- ↑ Jan Huisken, Didier YR Stainier: Even fluorescence excitation by multidirectional selective plane illumination microscopy (mSPIM) . In: Optics Letters . 32, No. 17, 2007, p. 2608. doi : 10.1364 / OL.32.002608 .
- ↑ Raju Tomer, Khaled Khairy, Fernando Amat, Philipp J Keller: Quantitative high-speed imaging of entire developing embryos with simultaneous multiview light-sheet microscopy . In: Nature Methods . 9, No. 7, June 3, 2012, pp. 755-763. doi : 10.1038 / nmeth.2062 .
- ↑ Uros Krzic, Stefan Gunther, Timothy E Saunders, Sebastian J Streichan, Lars Hufnagel: Multiview light-sheet microscope for rapid in toto imaging . In: Nature Methods . 9, No. 7, June 3, 2012, pp. 730-733. doi : 10.1038 / nmeth.2064 .
- ^ PJ Keller, AD Schmidt, J. Wittbrodt, EHK Stelzer: Reconstruction of Zebrafish Early Embryonic Development by Scanned Light Sheet Microscopy . In: Science . 322, No. 5904, November 14, 2008, pp. 1065-1069. doi : 10.1126 / science.1162493 .
- ^ FO Fahrbach, A. Rohrbach: A line scanned light-sheet microscope with phase-shaped self-reconstructing beams. In: Optics express. Volume 18, Number 23, November 2010, pp. 24229-24244, PMID 21164769 .
- ^ TA Planchon, L. Gao, DE Milkie, MW Davidson, JA Galbraith, CG Galbraith, E. Betzig: Rapid three-dimensional isotropic imaging of living cells using Bessel beam plane illumination. In: Nature methods. Volume 8, Number 5, May 2011, pp. 417-423, doi: 10.1038 / nmeth.1586 . PMID 21378978 .
- ↑ C. Dunsby: Optically sectioned oblique imaging plane by microscopy . In: Optics Express . 16, No. 25, 2008, p. 20306. doi : 10.1364 / OE.16.020306 .
- ↑ Zeno Lavagnino, Francesca Cella Zanacchi, Emiliano Ronzitti, Alberto Diaspro: Two-photon excitation selective plane illumination microscopy (2PE-SPIM) of highly scattering samples: characterization and application . In: Optics Express . 21, No. 5, 2013, p. 5998. doi : 10.1364 / OE.21.005998 .
- ↑ a b J. Capoulade, M. Wachsmuth, L. Hufnagel, M. Knop: Quantitative fluorescence imaging of protein diffusion and interaction in living cells. In: Nature Biotechnology . Volume 29, number 9, September 2011, pp. 835-839, doi: 10.1038 / nbt . 1928 . PMID 21822256 .
- ↑ a b c T. Wohland, X. Shi, J. Sankaran, EH Stelzer: Single plane illumination fluorescence correlation spectroscopy (SPIM-FCS) probes inhomogeneous three-dimensional environments. In: Optics express. Volume 18, No. 10, 2010, pp. 10627-10641, PMID 20588915 .
- ↑ a b Jan W Krieger, Anand P Singh, Nirmalya Bag, Christoph S Garbe, Timothy E Saunders, Jörg Langowski, Thorsten Wohland: Imaging fluorescence (cross-) correlation spectroscopy in live cells and organisms . In: Nature Protocols . tape 10 , no. 12 , November 5, 2015, p. 1948 , doi : 10.1038 / nprot.2015.100 ( uni-heidelberg.de [PDF]).
- ↑ Francesca Cella Zanacchi, Zeno Lavagnino, Michela Perrone Donnorso, Alessio Del Bue, Laura Furia, Mario Faretta, Alberto Diaspro: Live-cell 3D super-resolution imaging in thick biological samples . In: Nature Methods . 8, No. 12, October 9, 2011, pp. 1047-1049. doi : 10.1038 / nmeth.1744 .
- ↑ Jerome Mertz, Jinhyun Kim: Scanning light-sheet microscopy in the whole mouse brain with HiLo background rejection . In: Journal of Biomedical Optics . 15, No. 1, 2010, p. 016027. doi : 10.1117 / 1.3324890 .
- ↑ M. Friedrich, Q. Gan, V. Ermolayev, GS Harms: STED-SPIM: Stimulated emission depletion improves sheet illumination microscopy resolution. In: Biophysical Journal. Volume 100, Number 8, April 2011, pp. L43-L45, doi: 10.1016 / j.bpj.2010.12.3748 . PMID 21504720 . PMC 3077687 (free full text).
- ↑ Alexis Maizel, Daniel von Wangenheim, Fern n Federici, Jim Haseloff, Ernst HK Stelzer: High-resolution live imaging of plant growth in near physiological bright conditions using light sheet fluorescence microscopy . In: The Plant Journal . 68, No. 2, October 2011, pp. 377-385. doi : 10.1111 / j.1365-313X.2011.04692.x .
- ↑ Terrence F. Holekamp, Diwakar Turaga, Timothy E. Holy: Fast Three-Dimensional Fluorescence Imaging of Activity in Neural Populations by Objective-Coupled Planar Illumination Microscopy . In: Neuron . 57, No. 5, March 13, 2008, pp. 661-672. doi : 10.1016 / j.neuron.2008.01.011 .
- ↑ Y. Wu, A. Ghitani, R. Christensen, A. Santella, Z. Du, G. Rondeau, Z. Bao, D. Colon-Ramos, H. Shroff: Inverted selective plane illumination microscopy (iSPIM) enables coupled cell identity lineaging and neurodevelopmental imaging in Caenorhabditis elegans . In: Proceedings of the National Academy of Sciences . 108, No. 43, October 25, 2011, pp. 17708-17713. doi : 10.1073 / pnas.1108494108 .
- ^ Nobel Prize lecture by RA Zsigmondy (English): Properties of colloids (PDF; 108 kB), with an illustration and a brief explanation of the ultramicroscope
- ↑ Press release of LaVision Biotech ( Memento of 24 December 2013, Internet Archive ) (accessed on November 4, 2012)
- ↑ Carl Zeiss press release on the Lightsheet Z.1 light sheet microscope system (accessed on November 4, 2012)
- ^ PA Santi: Light Sheet Fluorescence Microscopy: A Review . In: Journal of Histochemistry & Cytochemistry . 59, No. 2, February 1, 2011, pp. 129-138. doi : 10.1369 / 0022155410394857 .
- ↑ OpenSPIM project webpage (accessed June 8, 2013)
- ↑ Peter G Pitrone, Johannes Schindelin, Luke Stuyvenberg, Stephan Preibisch, Michael Weber, Kevin W Eliceiri, Jan Huisken, Pavel Tomancak: OpenSPIM: an open-access light-sheet microscopy platform . In: Nature Methods . June 9, 2013. doi : 10.1038 / nmeth.2507 .
- ↑ The OpenSPIN project webpage (accessed June 8, 2013)
- ↑ Emilio J Gualda, Tiago Vale, Pedro Almada, Jos A Feij, Gabriel G Martins, Nuno Moreno: Open Spin Microscopy: an open-source integrated microscopy platform . In: Nature Methods . June 9, 2013. doi : 10.1038 / nmeth.2508 .
- ↑ PJ Verveer, J. Swoger, F. Pampaloni, K. Greger, M. Marcello, EH Stelzer: High-resolution three-dimensional imaging of large specimens with light sheet-based microscopy. In: Nature methods . Volume 4, No. 4, 2007, pp. 311-313, doi: 10.1038 / nmeth1017 , PMID 17339847 .
- ↑ Corinne Lorenzo, Céline Frongia, Raphael Jorand, Jérome Fehrenbach, Pierre Weiss, Amina Maandhui, Guillaume Gay, Bernard Ducommun, Valérie Lobjois: Live cell division dynamics monitoring in 3D large spheroid tumor models using light sheet microscopy . In: Cell Division . 6, No. 1, 2011, p. 22. doi : 10.1186 / 1747-1028-6-22 .
- ↑ Daisuke Takao, Atsushi Taniguchi, Takaaki Takeda, Seiji Sonobe, Shigenori Nonaka, Alexandre J. Kabla: High-Speed Imaging of Amoeboid Movements Using Light-Sheet Microscopy . In: PLoS ONE . tape 7 , no. 12 , December 5, 2012, p. e50846 , doi : 10.1371 / journal.pone.0050846 .