STED microscope
A STED microscope (STED = Stimulated Emission Depletion ) is a special form of light microscope , the resolution of which is not diffraction-limited. It can therefore still differentiate between structures that are significantly closer together than the limit of normal light microscopic resolution limit formulated by Ernst Abbe indicates. STED is one of several techniques that allow such increased resolution (see RESOLFT microscopy ).
Like all such methods, STED is a variant of fluorescence microscopy , so it requires the use of fluorescence dyes. These so-called fluorochromes can be excited by light of certain wavelengths and then spontaneously emit light again within a few nanoseconds over a range of longer, lower-energy wavelengths. The spontaneous radiation can be suppressed, however, if intensive light of one of these lower energy wavelengths is also radiated: Then the energy of the excited fluorochrome is artificially de-energized, resulting in stimulated emission . The name of the method comes from this depletion by means of stimulated emission.
In STED microscopy, a laser beam is focused into the specimen to excite the fluorochromes. At the same time, a ring of de-excitation light is placed in the outer areas of the focus so that spontaneous fluorescent light is only emitted from a central area that is smaller than the diffraction-limited excitation focus. The STED effect is initially limited to one point in the preparation. As with other laser scanning microscopes, this point is therefore scanned over the specimen in order to generate two- or three-dimensional images.
STED was theoretically described in 1994 by Stefan Hell and Jan Wichmann and implemented experimentally in 1999 by Stefan Hell and Thomas Klar . The STED microscope and Stefan Hell's group were awarded the German Future Prize for their results in 2006 . In October 2014, Stefan Hell was awarded the Nobel Prize in Chemistry for his work on STED . It is being further developed in the working group of Stefan Hell at the Max Planck Institute for Biophysical Chemistry in Göttingen. STED microscopes are also commercially available.
Basics
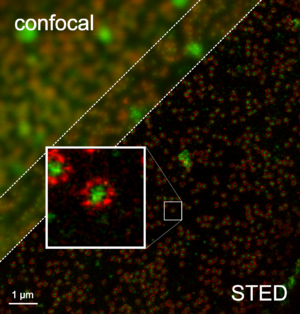
Due to diffraction , the resolution of conventional light microscopes is limited, a limit known as the Abbe limit : no details can be resolved that are smaller than about half the wavelength of the light used. Structures lying next to one another are only resolved from a distance of approx. 200 nm. The resolution for objects lying one behind the other (depth resolution) is even worse.
With the STED microscope, this Abbe limit is not overridden, but deliberately “tricked”: by switching off fluorescent dyes, the remaining still fluorescent area is greatly reduced (see below) and thus resolution below the Abbé limit is made possible. A resolution of 2.4 nm (lateral) could already be achieved.
A STED microscope is based on fluorescence laser scanning microscopes . In order to better understand how it works, it is necessary to address the most important aspects of fluorescence , stimulated emission and laser scanning microscopy :
Fluorescence and Stimulated Emission
In STED microscopy, so-called fluorescent dyes are used to mark individual areas of a specimen. Such dyes can be “excited” by light of certain wavelengths (colors): They absorb a photon and change to a more energetic state. From this state they can spontaneously return to the basic state after a short time by emitting a photon of greater wavelength (different color ). This spontaneous emission of light is called fluorescence. The fluorescent light can be separated from the excitation light by means of suitable color filters.
A stimulated dye molecule can return to its basic state not only through fluorescence but also through stimulated emission. This happens when the excited dye molecule is irradiated with light of approximately the same wavelength as that of the fluorescent light. The excited dye molecule can be stimulated to instantly transition to the ground state by emitting a photon of exactly the same wavelength. Spontaneous fluorescent light can then no longer be emitted, the molecule is no longer in the excited state. Fluorescent molecules can therefore be switched off by stimulated emission: if they are stimulated to emit light, they can no longer emit spontaneous fluorescent light. Spontaneous fluorescent light and light from the stimulated emission can e.g. B. separated by color filters.
Fluorescence laser scanning microscopy
For the examination in a fluorescence microscope, fluorescent dyes are brought to certain points on the specimen to be examined. If the preparation is now illuminated with light of a suitable wavelength, the dyes are excited to fluoresce and an image of the distribution of the dyes in the preparation is obtained. In the laser scanning microscope, the entire specimen is not illuminated at once, but a laser beam ("excitation beam") is only focused on a small point on the specimen. The sharp fluorescence remaining in this area is detected. By moving (scanning, rastering) the focus over the specimen, an image of the colored areas is created point by point. The size of the focus determines the maximum fineness of the details that can just barely be resolved (perceived separately) in the specimen. Because of the diffraction , the focus cannot be chosen arbitrarily small. A laser beam cannot be focused on a spot smaller than about half its wavelength.
Functional principle of the STED microscope
With a STED microscope, better resolution is possible than with a conventional laser scanning microscope: the area from which fluorescence is emitted is made significantly smaller than the area that is illuminated by the laser beam. This is achieved by specifically switching off the dye molecules in the outer area of the focus. For this purpose, the specimen is not only illuminated with the focused excitation beam (left picture), but also with a second laser beam, the “switch-off beam”. This switch-off beam is given a ring-shaped profile in focus (middle picture). In the middle, i.e. where the excitation beam has its maximum brightness, the switch-off beam is completely dark. The switch-off beam does not affect the fluorescent dyes in the middle. But it switches off the fluorescent dyes in the outer area of the excitation focus by stimulated emission (see above); the dye molecules in the outer area remain dark, although they are illuminated by the excitation laser. Therefore, only the dye molecules shine exactly from the center (right picture). The minimum diameter of the excitation beam is just as diffraction-limited as the central dark field of the switch-off beam. However, a few photons of the switch-off beam are sufficient to stimulate the emission of a larger number of excited states; In addition, the intensity of the switch-off beam can be selected to be higher than that of the excitation beam. As a result, the central area that is not switched off is much smaller than the area illuminated with the excitation laser (see line profiles on the right). When the specimen is scanned, a luminous spot is recorded that is much smaller than in a normal laser scanning microscope. Therefore one can resolve finer details. To obtain a complete image, the specimen is scanned point by point.
The size of the resulting light spot decreases more and more as the intensity of the switch-off beam increases. This means that the resolution increases the brighter the switch-off beam is; the achievable resolution is in principle not limited. Before the invention of STED microscopy, the problem existed that the excitation beam could not be focused as small as desired because of the Abbe's diffraction limit . So you always excite all the molecules that are currently in focus and therefore cannot decide which molecule the fluorescence is coming from. Therefore, structures that are smaller than the size of the laser focus could not be distinguished.
Applications
A significant problem regardless of which light microscopic technique is the lack of contrast of cell components. For a long time, therefore, fluorescent molecules have been used. B. with genetic engineering methods or by means of antibodies can be selectively attached to certain molecules of a cell. For example, dyes can only be grown on mitochondria . If you now illuminate a point of the cell prepared in this way with a focused laser beam and receive fluorescence from there, then there were dye molecules and thus mitochondria at precisely this point. To obtain a complete image, the specimen is scanned point by point. All preparations that can be marked with fluorescent dyes can be examined in a STED microscope. Unlike electron microscopes, no vacuum and no thin cuts are required. That is why living cells can also be observed.
In contrast to scanning probe microscopy , a near-field method, STED microscopy is a far-field technique. So it is not limited to examining surfaces. You can also examine the inside of cells, for example. It is also possible to observe fast dynamic processes with up to 200 images per second.
literature
- Marcus Dyba, Stefan W. Hell: Focal spots of size lambda / 23 open up far-field florescence microscopy at 33 nm axial resolution . In: Physical Review Letters . Vol. 88, No. 16 , 2002, pp. 163901 , doi : 10.1103 / PhysRevLett.88.163901 .
- Katrin I. Willig, Silvio O. Rizzoli, Volker Westphal, Reinhard Jahn, Stefan W. Hell: STED-microscopy reveals that synaptotagmin remains clustered after synaptic vesicle exocytosis . In: Nature . Vol. 440, 2005, pp. 935-939 , doi : 10.1038 / nature04592 .
- Stefan W. Hell: Microscopy and its focal switch . In: Nature Methods . Vol. 6, No. 1 , 2009, p. 24-32 , doi : 10.1038 / nmeth.1291 .
- Stefan W. Hell: Far-Field Optical Nanoscopy . In: Science . Vol. 316, 2007, pp. 1153-1158 , doi : 10.1126 / science.1137395 .
Web links
- Department NanoBiophotonics at the Max Planck Institute for Biophysical Chemistry (English)
- Detailed information and pictures of STED microscopy (PDF file; 426 kB)
- German Future Prize 2006: Light microscopy in unprecedented sharpness - background material
- STED - microscopy beyond optical limits, film on the YouTube channel of the Max Planck Society
Individual evidence
- ↑ Stefan W. Hell and Jan Wichmann: Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy . In: Optics Letters . tape 19 , no. 11 , 1994, pp. 780-782 , doi : 10.1364 / OL.19.000780 .
- ↑ Thomas A. Klar , Stefan W. Hell: Subdiffraction resolution in far-field fluorescence microscopy . In: Optics Letters . Vol. 24, No. 14 , 1999, p. 954-956 , doi : 10.1364 / OL.24.000954 .
- ^ The Nobel Prize in Chemistry 2014
- ↑ SPIEGEL-Online of October 9, 2014: 'Razor-sharp view into the heart of life' ; Retrieved October 9, 2014
- ↑ WELT-Online of October 8, 2014: 'Nobel Prize for the developer of the supermicroscope' ; Retrieved October 9, 2014
- ↑ Press release of the MPI Göttingen from October 8, 2014 ; Retrieved October 9, 2014
- ↑ The TCS STED has been on the market since 2007 and delivers a resolution below 100 nm. The TCS STED CW has been available with a resolution below 80 nm since 2009 .
- ↑ D. Wildanger, BR Patton, H. Schill, L. Marseglia, JP Hadden, S. Knauer, A. Schönle, JG Rarity, JL O'Brien, SW Hell, JM Smith :: Solid Immersion Facilitates Fluorescence Microscopy with Nanometer Resolution and Sub-Angstrom Emitter Localization. In: Advanced Materials . 2012, doi : 10.1002 / adma.201203033 .
- ^ Stefan W. Hell: Far-Field Optical Nanoscopy . In: Science . Vol. 316, 2007, pp. 1153-1158 , doi : 10.1126 / science.1137395 .
- ↑ Volker Westphal, Silvio O. Rizzoli, Marcel A. Lauterbach, Dirk Kamin, Reinhard Jahn, Stefan W. Hell: Video-Rate Far-Field Optical Nanoscopy Dissects Synaptic Vesicle Movement . In: Science . Vol. 320, 2008, ISSN 0036-8075 , pp. 246-249 , doi : 10.1126 / science.1154228 .
- ↑ Volker Westphal, Marcel A. Lauterbach, Angelo Di Nicola, Stefan W. Hell: Dynamic far-field fluorescence nanoscopy . In: New Journal of Physics . tape 9 , 2007, p. 435 ff ., doi : 10.1088 / 1367-2630 / 9/12/435 .
- ↑ Marcel A. Lauterbach, Chaitanya K. Ullal, Volker Westphal, and Stefan W. Hell: Dynamic Imaging of Colloidal-Crystal Nanostructures at 200 Frames per Second . In: Langmuir . tape 26 , 2010, p. 14400-14404 ., Doi : 10.1021 / la102474p .

