Lupine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
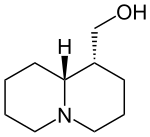
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Lupine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 19 NO | ||||||||||||||||||
| Brief description |
white to light brown solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 169.26 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
68.5-69.2 ° C |
||||||||||||||||||
| boiling point |
160-164 ° C at 4 h Pa |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Lupinine is a bitter-tasting substance from the group of substances of quinolizidine - alkaloids .
Occurrence
Lupinine occurs, among other things, in the seeds of the wolf bean ( Lupinus polyphyllus , lupins ) from North America .
Extraction and presentation
Lupinine can be obtained from natural sources as well as synthetically.
properties
toxicology
Lupinine can cause death from respiratory paralysis.
Physical Properties
Optical activity α: −19.5 ° (10 g / l, measured in ethanol, wavelength: 589 nm, resonance frequency: 509 THz)
literature
- Morley, Knight, Share: Journal of the Chemical Society . Perkin Transactions 1 , (20), 1994, 2903-2908
Individual evidence
- ↑ a b c data sheet (-) - Lupinine, 97% at AlfaAesar, accessed on December 7, 2019 ( PDF )(JavaScript required) .
- ↑ a b The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, pp. 972-973, ISBN 978-0-911910-00-1 .
- ↑ Entry on lupine. In: Römpp Online . Georg Thieme Verlag, accessed on April 14, 2016.
- ^ A b Albert Gossauer: Structure and Reactivity of Biomolecules , Verlag Helvetica Chimica Acta, Zurich, 2006, p. 250, ISBN 978-3-906390-29-1 .

