meso- zeaxanthin
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
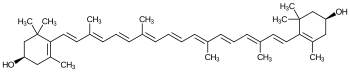
|
||||||||||
| General | ||||||||||
| Surname | Meso-zeaxanthin | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 40 H 56 O 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 568.87 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
meso -zeaxanthin [(3 R , 3 ′ S ) -zeaxanthin] is a carotenoid of the xanthophyll class and is one of three stereoisomers of zeaxanthin . Of these three stereoisomers, meso -zeaxanthinoccurssecond most frequently in nature, after (3 R , 3 ′ R ) -zeaxanthin, which is produced by plants and algae. To date, meso- zeaxanthin has been detected in certain tissues of marine fish, but also in the yellow spot (Latin: macula lutea ) of the retina of the human eye.
Occurrence
Carotenoids are necessary for animal life, but animals cannot make them themselves. Therefore, animals ingest carotenoids with their food. For herbivores the sources are vegetable and algae food, for carnivores herbivores in turn serve as a corresponding source.
Occurrence in food

It is now generally accepted that meso- zeaxanthin is not found in plants themselves, but is found in marine fish. It was originally believed that meso -zeaxanthin was not derived from food and would be formed in the macula (the central part of the retina) from retinal lutein (another xanthophyll found in human nutrition), but this work has since been refuted. Nolan et al. a. (2013) showed that meso- zeaxanthin is found in the skin of trout, sardines and salmon and in the meat of trout. This is consistent with the work of Maoka from 1986. In another publication by Nolan and co-workers, the three stereoisomers of zeaxanthin in the meat of two species of trout were quantified. This was the first study to investigate the amount of meso- zeaxanthin in a common food. It was calculated that a person ingests 0.2 mg of natural meso- zeaxanthin from an average rainbow trout (approx. 200 g) . In addition, canned sardines can also be considered a common source of meso- zeaxanthin in humans, as these canned foods contain a significant proportion of sardine skin. As early as 2002, Khachick et al. Reported that the liver of Japanese quail (Coturnix japonica) and frog plasma contained meso- zeaxanthin. Frog legs are a relatively common food and delicacy in France.

It is also possible that meso -zeaxanthin is formed from other carotenoids that are ingested with food, as carotenoids are known to be converted into each other for specific physiological functions. For example, it has been suggested that meso -zeaxanthin is derived from astaxanthin in the skin of trout; and meso- zeaxanthin in the yellow spot of primates is at least partially made from lutein.
Macula
Meso- zeaxanthin, lutein and (3 R , 3 ′ R ) -zeaxanthin are the most common carotenoids in the macula lutea , which occur in a ratio of 1: 1: 1 and are collectively referred to as macular pigment (MP). Meso- zeaxanthin is concentrated in the center of the macula, where it makes up about 50% of the MP, while lutein dominates in the peripheral macula.
Poultry products
Broilers are yellow when they have been fed food containing carotenoids, as the carotenoids accumulate in the animals' skin and subcutaneous fat. Carotenoid storage is also one reason for the egg yolk's yellow color. For this reason, poultry producers add carotenoids to the feed in order to make the end product more attractive to the consumer and to support the health of the animals (typically lutein, zeaxanthin, canthaxanthin and 8′-apo-β-caroten-8′-al ). It is believed that lutein and zeaxanthin synergistically enhance the yellow hue, with zeaxanthin being stronger than lutein due to its larger chromophore . That's why some companies use marigold extract, in which some of the lutein has been converted into zeaxanthin, to feed both carotenoids to the broilers and hens. The isomer of zeaxanthin, which is produced from lutein during the manufacturing process, is meso -zeaxanthin due to the technology used (see below). Therefore, meso- zeaxanthin was found in eggs from Mexico and California.
Properties and biological importance
Among the three macular carotenoids (lutein, zeaxanthin and meso -zeaxanthin), meso -zeaxanthin is the most powerful antioxidant , but the combination of macular carotenoids showed the greatest antioxidant potential when compared to individual carotenoids at the same concentration. This seems to explain why the human macula contains only these three of the approximately 700 carotenoids found in nature. It has also been shown that this combination of carotenoids leads to light filtering of the short-wave blue light in the macula. This is important because the short-wave light hitting the macula otherwise leads to chromatic aberration and light scattering, phenomena that negatively affect the visual function and lead to reduced contrast vision. meso -zeaxanthin is in the ideal anatomical position and has antioxidant and light-filtering properties to protect the macula and strengthen eyesight.
use
Carotenoids such as zeaxanthin and lutein are used in combination with other substances in food supplements as micronutrients for the eyes. In two clinical studies ( AREDS -1 and -2) with more than 7700 participants in 2001 and 2013, the combined intake of antioxidants such as vitamin C , vitamin E , zinc and copper oxide with β-carotene or with the macular carotenoids lutein and zeaxanthin examined for the progression of AMD.
The use of meso- zeaxanthin specifically in the treatment of age-related macular degeneration is being investigated.
There are no health claims under the European Health Claims Regulation .
Industrial manufacture
Industrially, meso -zeaxanthin made of lutein, which from petals of Marigold is recovered. The process consists of saponification at high temperatures and a high base concentration, which leads to the isomerization of the 4'-5'-carbon double bond to the 5'-6 'position. This converts the ɛ ring of lutein into a β ring and converts lutein into meso -zeaxanthin. The stereochemistry of this zeaxanthin is determined by the position of the hydroxyl group at the 3 'position, which leads to the S configuration of the resulting zeaxanthin isomer. Therefore, the stereoisomer (3 R. 3 ′ S ) -zeaxanthin (i.e. meso -zeaxanthin) is produced by this process . The conditions of this saponification can be influenced in such a way that the conversion rate of lutein to meso- zeaxanthin is increased or decreased.
Animal safety studies
If a substance is to be used commercially for human consumption, its safety must be assessed. First of all, it must be shown that the substance is harmless to animal health, even when administered in doses that are significantly higher than the usual daily intake. The substance can then be examined in human studies.
Toxicity studies of meso -zeaxanthin have been performed by various research groups, all of which have confirmed the safety of this compound.
The results of these studies can be summarized as follows:
- Chang et al. a. showed that the NOAEL (No Observed-Adverse-Effect Level) was higher than the dose of 200 mg / kg body weight per day and thus higher than the intake with dietary supplements, which was typically less than 0.5 mg / kg body weight per day lies. The absence of mutagenicity was confirmed by the same study using the Ames test .
- Xu et al. a. concluded from a 90-day nutrition study in rats that meso -zeaxanthin has no acute toxicity and no genotoxicity ; and that the supply of meso- zeaxanthin is safe at a dose of 300 mg / kg body weight per day. The authors used a 100-fold safety factor and calculated an ADI (Acceptable Daily Intake) of 3 mg / kg body weight per day for meso- zeaxanthin.
- Thurnham et al. a. showed in rats that amounts of 2, 20 and 200 mg meso- zeaxanthin per kg body weight per day for 13 weeks did not lead to any undesirable effects on the health of the animals. This means that the NOAEL is at least 200 mg meso- zeaxanthin / kg body weight per day, which is at least 1400 times higher than the typical intake with food supplements. Investigations on genotoxicity showed the safety of 10 to 5000 µg meso- zeaxanthin per bacterial culture plate in the so-called Ames test and did not lead to an increased mutation rate in five bacterial test strains.
In summary, it can be concluded that the NOAEL for meso- zeaxanthin is much higher than the intake from conventional foods.
In 2011 the US “ Food and Drug Administration ” recognized the GRAS status (GRAS: Generally Recognized As Safe ) of meso-zeaxanthin on the basis of a proposal by a US company on the status of meso-zeaxanthin (plus L and Z).
Studies in Man
The first study to examine the effects of meso-zeaxanthin was carried out by Professors Bone and Landrum in Miami, Florida. This investigation confirmed that meso-zeaxanthin was effectively absorbed into the serum and that the macular pigment density increased significantly in the group with supplementation. No such increase was observed in the placebo group.
In another study in Northern Ireland, 19 individuals took a supplement that also consisted of all three macular carotenoids (including meso-zeaxanthin ) over a period of 22 days. The results indicated that meso-zeaxanthin was ingested. At the "Institute of Vision Research" of the "Waterford Institute of Technology" several studies ("Meso-zeaxanthin Ocular Supplementation Trials" [MOST]) were carried out to assess the safety, the effect on the macular pigment density and the serum carotenoid concentration in To examine individuals with and without AMD after supplementation with all three macular carotenoids (particularly meso-zeaxanthin ). These studies confirmed the safety of macular carotenoids for human consumption. Many biological tests were carried out to check kidney and liver function, as well as the lipid profile, blood count and inflammation markers. The MOST studies also showed a statistically significant increase in the serum concentrations of meso-zeaxanthin and lutein compared to the initial value. Significant increases in central macular pigment were observed after just two weeks of supplementation. In addition, in patients who had an atypical distribution of macular pigment in the eye, the normal pigment profile was restored (i.e., they had the high concentration of pigment in the center of the macula). This happened after taking a predominantly meso-zeaxanthin -containing preparation for eight weeks, whereas this did not occur in the group with the preparation without meso-zeaxanthin .
The main results from the MOST studies in patients with AMD were published in 2013 and 2015. The publications of these studies concluded: "The enrichment of the macular pigment density via its spatial distribution profile and the enhancement of the contrast sensitivity were best achieved after supplementation with a formulation that contained high doses of meso-zeaxanthin in combination with lutein and zeaxanthin." The 2015 publication concludes with reference to age-related macular degeneration: "The inclusion of meso-zeaxanthin in a formulation appears to provide a benefit in increasing macular pigment density and in improving contrast sensitivity in patients with early AMD. An important and new finding is based based on the observation that continued macular carotenoid supplementation for three years in patients with early AMD appears necessary for a maximum increase in macular pigment density and for optimized contrast sensitivity.
Web links
- Jennifer Grebow: Fierce Debate over Zeaxanthin Isomers. NutritionalOutlook.com, UBM Medica Network, June 24, 2014.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ TE De Ville, MB Hursthouse, SW Russell, BCL Weedon: Absolute configuration of carotenoids. In: pubs.rsc.org. Retrieved March 7, 2016 .
- ↑ a b Takashi Maoka, Akihiro Arai, Minoru Shimizu, Takao Matsuno: The first isolation of enantiomeric and Meso-zeaxanthin in nature . In: Comparative Biochemistry and Physiology Part B: Comparative Biochemistry . tape 83 , no. 1 , January 1, 1986, p. 121-124 , doi : 10.1016 / 0305-0491 (86) 90341-X .
- ↑ a b Richard A. Bone, John T. Landrum, Larry M. Friedes, Christina M. Gomez, Mark D. Kilburn: Distribution of Lutein and Zeaxanthin Stereoisomers in the Human Retina . In: Experimental Eye Research . tape 64 , no. 2 , February 1, 1997, p. 211-218 , doi : 10.1006 / exer.1996.0210 .
- ^ RA Bone, JT Landrum, GW Hime, A. Cains, J. Zamor: Stereochemistry of the human macular carotenoids . In: Investigative Ophthalmology & Visual Science . tape 34 , no. 6 , May 1, 1993, pp. 2033-2040 , PMID 8491553 .
- ↑ a b Prakash Bhosale, Bogdan Serban, Da You Zhao, Paul S. Bernstein: Identification and Metabolic Transformations of Carotenoids in Ocular Tissues of the Japanese Quail Coturnix japonica † . In: Biochemistry . tape 46 , no. 31 , July 14, 2007, p. 9050-9057 , doi : 10.1021 / bi700558f , PMID 17630780 , PMC 2531157 (free full text).
- ↑ a b c Helen M. Rasmussen, Tawanda Muzhingi, Emily MR Eggert, Elizabeth J. Johnson: Lutein, zeaxanthin, meso-zeaxanthin content in egg yolk and their absence in fish and seafood . In: Journal of Food Composition and Analysis . tape 27 , no. 2 , September 1, 2012, p. 139–144 , doi : 10.1016 / j.jfca.2012.04.009 .
- ↑ JM Nolan, K. Meagher, S. Kashani, S. Beatty: What is meso-zeaxanthin, and where does it come from? In: Eye . tape 27 , no. 8 , August 1, 2013, p. 899-905 , doi : 10.1038 / eye.2013.98 , PMID 23703634 , PMC 3740325 (free full text).
- ↑ Prado-Cabrero, A., Beatty, S., Stack, J., Howard, A., Nolan, JM: Quantification of zeaxanthin stereoisomers and lutein in trout flesh using chiral high-performance liquid chromatography-diode array detection. In: Journal of Food Composition and Analysis. doi : 10.1016 / j.jfca.2016.05.004 .
- ↑ Khachik, F., de Moura, FF, Zhao, DY, Aebischer, CP, Bernstein, PS: Transformations of Selected Carotenoids in Plasma, Liver, and Ocular Tissues of Humans and in Nonprimate Animal Models. In: Investigative Ophthalmology & Visual Science . tape 43 , no. 11 , November 1, 2002, ISSN 1552-5783 , p. 3383-3392 , PMID 12407147 .
- ↑ Katharina Schiedt, Max Vecchi, Ernst Glinz: Astaxanthin and its metabolites in wild rainbow trout (Salmo gairdneri R.) . In: Comparative Biochemistry and Physiology Part B: Comparative Biochemistry . tape 83 , no. 1 , January 1, 1986, p. 9-12 , doi : 10.1016 / 0305-0491 (86) 90324-X .
-
↑ This figure shows the distribution of macular pigments in the healthy human retina: the carotenoids meso- zeaxanthin, zeaxanthin and lutein. The data on the localization of the carotenoids come from the following sources:
Published studies:- RA Bone, JT Landrum, Z. Dixon, Y. Chen, CM Lerena: Lutein and zeaxanthin in the eyes, serum and diet of human subjects. In: Experimental Eye Research. 71, 2000, pp. 239-245.
- JM Nolan, MC Akkali, J. Loughman, AN Howard, S. Beatty: Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment. In: Exp Eye Res. 101, 2012, pp. 9-15.
- S. Sabour-Pickett, S. Beatty, E. Connolly et al: Supplementation with three different macular carotenoid formulations in patients with early age-related macular degeneration. In: Retina. 34, 2014, pp. 1757-1766.
- KO Akuffo, JM Nolan, AN Howard et al .: Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration. In: Eye (Lond). 29, 2015, pp. 902-912.
- G. Britton, S. Liaaen-Jensen, H. Pfander: Interpretation of Stereo Ocular Angiography: Retinal and Choroidal Anatomy. In: Springer Science and Business Media. 2009, p. 301.
- M. Yanoff: Ocular Pathology. Elsevier Health Sciences. 2009, p. 393.
- RG Small: The Clinical Handbook of Ophthalmology. CRC Press, 1994, p. 134.
- GA Peyman, SA Meffert, F. Chou, MD Conway: Vitreoretinal Surgical Techniques. CRC Press, 2000, p. 6.
- ↑ a b M. D. Torres-Cardona, J. Torres-Quiroga: Process for the isomerization of lutein. Industrial Organica, SA de CV, Monterrey, Mexico, US 1996.
- ↑ Binxing Li, Faisal Ahmed, Paul S. Bernstein: Studies on the singlet oxygen scavenging mechanism of human macular pigment . In: Archives of Biochemistry and Biophysics (= Carotenoids ). tape 504 , no. 1 , December 1, 2010, p. 56–60 , doi : 10.1016 / j.abb.2010.07.024 , PMID 20678467 , PMC 2957523 (free full text).
- ↑ Richard A. Bone, John T. Landrum, Yisi Cao, Alan N. Howard, Francesca Alvarez-Calderon: Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin . In: Nutrition & Metabolism . tape 4 , January 1, 2007, p. 12 , doi : 10.1186 / 1743-7075-4-12 , PMID 17498306 , PMC 1872023 (free full text).
- ↑ Eithne E. Connolly, Stephen Beatty, James Loughman, Alan N. Howard, Michael S. Louw: Supplementation with all three macular carotenoids: response, stability, and safety . In: Investigative Ophthalmology & Visual Science . tape 52 , no. 12 , November 1, 2011, p. 9207-9217 , doi : 10.1167 / iovs.11-8025 , PMID 21979997 .
- ↑ Sarah Sabour-Pickett, Stephen Beatty, Eithne Connolly, James Loughman, Jim Stack: Supplementation with three different macular carotenoid formulations in patients with early age-related macular degeneration . In: Retina (Philadelphia, Pa.) . tape 34 , no. 9 , September 1, 2014, p. 1757-1766 , doi : 10.1097 / IAE.0000000000000174 , PMID 24887490 .
- ↑ Eithne E. Connolly, Stephen Beatty, David I. Thurnham, James Loughman, Alan N. Howard: Augmentation of macular pigment following supplementation with all three macular carotenoids: an exploratory study . In: Current Eye Research . tape 35 , no. 4 , April 1, 2010, p. 335-351 , doi : 10.3109 / 02713680903521951 , PMID 20373901 .
- ↑ EU Register on Nutrition and Health Claims - zeaxanthin
- ↑ AG Andrewes: Isomerization of epsilon-carotene to beta-carotene and lutein to zeaxanthin of. In: Acta Chemica Scandinavica. B 28 (1), 1974, pp. 137-138.
- ^ AG Andrewes, GL Borch, S. Liaaen-Jensen: Carotenoids of Higher Plants 7. * On the Absolute Configuration of Lutein. In: Acta Chemica Scandinavica. B 28 (1), 1974, pp. 139-140.
- ↑ Sunil Kumar TK, Sherena P. Abdulkadir, Shankaranarayana Madapura Lingappiah: Xanthophyll composition containing trans, meso-zeaxanthin, trans, R, R-zeaxanthin and trans, R, R-lutein useful for nutrition and health care and a process for its preparation . 2012.
- ↑ CJG Chang: Thirteen-week oral (gavage) toxicity of meso-zeaxanthin in Han Wistar rats with a 4-week recovery. 2006.
- ↑ David I. Thurnham, Alan N. Howard: Studies on meso-zeaxanthin for potential toxicity and mutagenicity . In: Food and Chemical Toxicology . tape 59 , September 1, 2013, p. 455-463 , doi : 10.1016 / j.fct.2013.06.002 .
- ↑ Xinde Xu, Lihua Zhang, Bin Shao, Xiaoxia Sun, Chi-Tang Ho: Safety evaluation of meso-zeaxanthin . In: Food Control . tape 32 , no. 2 , August 1, 2013, p. 678-686 , doi : 10.1016 / j.foodcont.2013.02.007 .
- ^ RA Bone, JT Landrum, Y. Cao, AN Howard, F. Alvarez-Calderon: Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. In: Nutrition & metabolism. Volume 4, 2007, p. 12, doi: 10.1186 / 1743-7075-4-12 , PMID 17498306 , PMC 1872023 (free full text).
- ^ EE Connolly, S. Beatty, J. Loughman, AN Howard, MS Louw, JM Nolan: Supplementation with all three macular carotenoids: response, stability, and safety. In: Investigative ophthalmology & visual science. Volume 52, Number 12, November 2011, pp. 9207-9217, doi: 10.1167 / iovs.11-8025 , PMID 21979997 .
- ^ S. Sabour-Pickett, S. Beatty, E. Connolly, J. Loughman, J. Stack, A. Howard, R. Klein, BE Klein, SM Meuer, CE Myers, KO Akuffo, JM Nolan: Supplementation with three different macular carotenoid formulations in patients with early age-related macular degeneration. In: Retina (Philadelphia, Pa.). Volume 34, Number 9, September 2014, pp. 1757-1766, doi: 10.1097 / IAE.0000000000000174 , PMID 24887490 .
- ↑ EE Connolly, S. Beatty, DI Thurnham, J. Loughman, AN Howard, J. Stack, JM Nolan: Augmentation of macular pigment following supplementation with all three macular carotenoids: an exploratory study. In: Current eye research. Volume 35, Number 4, April 2010, pp. 335-351, doi: 10.3109 / 02713680903521951 , PMID 20373901 .
- ↑ John M. Nolan, Mukunda C. Akkali, James Loughman, Alan N. Howard, Stephen Beatty: Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment . In: Experimental Eye Research . 101, August 1, 2012, ISSN 1096-0007 , pp. 9-15. doi : 10.1016 / j.exer.2012.05.006 . PMID 22652506 .
- ^ S. Sabour-Pickett, S. Beatty, E. Connolly, J. Loughman, J. Stack, A. Howard, R. Klein, BE Klein, SM Meuer, CE Myers, KO Akuffo, JM Nolan: Supplementation with three different macular carotenoid formulations in patients with early age-related macular degeneration. In: Retina (Philadelphia, Pa.). Volume 34, Number 9, September 2014, pp. 1757-1766, doi: 10.1097 / IAE.0000000000000174 , PMID 24887490 .
- ↑ KO Akuffo, JM Nolan, AN Howard, R. Moran, J. Stack, R. Klein, BE Klein, SM Meuer, S. Sabour-Pickett: Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration . In: Eye (London, England) . 29, No. 7, July 1, 2015, ISSN 1476-5454 , pp. 902-912. doi : 10.1038 / eye.2015.64 . PMID 25976647 . PMC 4506345 (free full text).


