Methyl fluorosulfonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
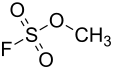
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Methyl fluorosulfonate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | CH 3 FO 3 S | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 114.09 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.45 g cm −3 |
|||||||||||||||
| Melting point |
−95 ° C |
|||||||||||||||
| boiling point |
93 ° C |
|||||||||||||||
| Refractive index |
1.3325 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Methyl fluorosulfonate (also known as magic methyl ) is a methylation reagent .
properties
Methyl fluorosulfonate is produced by distilling an equimolar mixture of fluorosulfonic acid and dimethyl sulfate . It is about four orders of magnitude more reactive than methyl iodide . In biological tissues, the methylation of biomolecules by methyl fluorosulfonate has an acutely toxic effect ( LC 50 (rat) ~ 5 ppm), mutagenic and causes irritation of the respiratory tract and pulmonary edema .
| property | value |
|---|---|
| Hydrogen bond acceptor | 3 |
| Hydrogen bond donor | 0 |
| Octanol-water partition coefficient | -0.3 ALogP |
| Solubility ( log solubility ) | -0.9 log mol / L |
| Polar Surface Area ( PSA ) | 51.8 Å 2 |
See also
- Methyl trifluoromethyl sulfonate , a similarly potent, somewhat more commonly used methylating agent
Individual evidence
- ↑ a b c d e f Lide: 1998 Freshman Achievement Award . CRC Press, 1998, ISBN 0-8493-0594-2 , pp. 3–352 ( limited preview in Google Book search).
- ↑ TCI: Methyl Fluorosulfonate (stabilized with KF) , accessed April 8, 2014
- ↑ Hite, M., Rinehart, W .; Braun, W .; Peck, H .: Acute toxicity of methyl fluorosulfonate (Magic Methyl) . In: AIHA Journal . 40, No. 7, 1979, pp. 600-603. doi : 10.1080 / 00028897708984416 . PMID 484483 .
- ↑ J. Ashby, D. Anderson, JA Styles: The potential carcinogenicity of methyl fluorosulphonate (CH 3 OSO 2 F; Magic Methyl). In: Mutation Research. Volume 51, Number 2, August 1978, pp. 285-287, PMID 211408 .
- ↑ Arup K. Ghose, Vellarkad N. Viswanadhan John J. Wendoloski: Prediction of Hydrophobic (lipophilic) Properties of Small Organic Molecules Using fragmental Methods: An Analysis of AlogP and CLOGP Methods. In: The Journal of Physical Chemistry A. 102, 1998, pp. 3762-3772, doi : 10.1021 / jp980230o .
- ↑ Arup K. Ghose, Vellarkad N. Viswanadhan John J. Wendoloski: Prediction of Hydrophobic (lipophilic) Properties of Small Organic Molecules Using fragmental Methods: An Analysis of AlogP and CLOGP Methods. In: The Journal of Physical Chemistry A. 102, 1998, p. 3762, doi : 10.1021 / jp980230o .
- ↑ IV Tetko, VY Tanchuk, TN Kasheva, AE Villa: Estimation of wässrige solubility of chemical compounds using e-state indices. In: Journal of chemical information and computer sciences. Volume 41, Number 6, 2001 Nov-Dec, pp. 1488-1493, PMID 11749573 .
- ↑ P. Ertl, B. Rohde, P. Selzer: Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. In: Journal of medicinal chemistry. Volume 43, Number 20, October 2000, pp. 3714-3717, ISSN 0022-2623 . PMID 11020286 .
- ↑ Roger W. Alder, Justin GE Phillips, Lijun Huang, Xuefei Huang: Methyltrifluoromethanesulfonate Encyclopedia of Reagents for Organic Synthesis, 2005, John Wiley & Sons, doi : 10.1002 / 047084289X.rm266m.pub2 .


