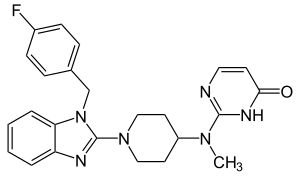
Mizolastine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Mizolastine | |||||||||||||||
| other names |
Latin : Mizolastinum |
|||||||||||||||
| Molecular formula | C 24 H 25 FN 6 O | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 432.49 g · mol -1 | |||||||||||||||
| Melting point |
217 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Mizolastine is a drug , known as H 1 - antihistamine especially in symptomatic treatment of allergic rhinitis , allergic conjunctivitis and urticaria applies.
chemistry
Mizolastine is a benzimidazole derivative and structurally it is similar to astemizole . This active ingredient is a specific antagonist that blocks the histamine H 1 receptors . It does not affect adrenaline , acetylcholine or serotonin receptors . Mizolastine was developed in 1998 by the pharmaceutical company sanofi-aventis , which, however, also sold the patent rights to Schwarz Pharma .
effect
Mizolastine is intended for oral intake only, is absorbed relatively quickly, so that a maximum plasma level is reached after approx. 60 to 90 minutes and the effect lasts over 24 hours thanks to the relatively long elimination half-life. The affinity of mizolastine for the H 1 receptors is 10 times greater than that of cetirizine and about 20 times greater than that of loratadine .
Due to the lack of potential to cross the blood-brain barrier, it only acts on peripheral H 1 receptors and, in contrast to other antihistamines, has almost no sedating effect, although temporary symptoms of tiredness can still occur.
Apparently mizolastine also has the ability to inhibit the synthesis of leukotrienes , which is why it is said to have anti-inflammatory properties. In some cases, influences on the QT interval were observed, but so far neither definitive effects on the QT interval nor arrhythmias in connection with mizolastine have been observed.
Since cytochrome P450 isoenzymes are involved in the metabolism of mizolastine, interactions occur when CYP3A4 inhibitors are administered at the same time; ketoconazole and erythromycin are therefore contraindicated.
pharmacy
In clinical studies, mizolastine showed roughly comparable effectiveness to cetirizine and loratadine. It is available in film-coated tablets of 10 mg and requires a prescription in Germany. An application in children and pregnant and breastfeeding women is not intended due to a lack of studies.
The currently lower number of prescriptions for mizolastine preparations is probably due to the comparatively higher price and the fact that patent protection is still active and therefore generics can only be approved in the future. The two original preparations were sold under the names Mizollen (Synthélabo) and Zolim (Schwarz Pharma) - both brands are now owned by Sanofi .
See also
Individual references / comments
- ↑ Entry on mizolastine. In: Römpp Online . Georg Thieme Verlag, accessed on July 16, 2019.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.