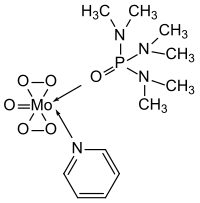
MoOPH
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | MoOPH | ||||||
| other names |
Oxodiperoxymolybdenum pyridine hexamethylphosphoramide |
||||||
| Molecular formula | C 11 H 23 MoN 3 O 6 P | ||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 324.29 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
103–105 ° C (decomposition) |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
MoOPH (or oxodiperoxymolybdenum pyridine hexamethylphosphoric acid triamide) is a molybdenum complex which Edwin Vedejs presented as a reagent for organic synthesis. The complex is used for the oxidation of carbanions .
application
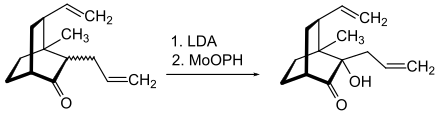
MoOPH is an oxidizing agent for carbanions such as B. enolate ions . In the example shown, an enolate ion is generated with the help of lithium diisopropylamide (LDA) and hydroxylated to α-hydroxyketone ( acyloin ) by reaction with MoOPH :
Since the hexamethylphosphoric acid triamide (HMPT) used as a ligand is classified as carcinogenic , an analogue with the ligand dimethylpropyleneurea (DMPU), the so-called MoOPD (oxodiperoxymolybdenum-pyridine-dimethylpropyleneurea), was introduced.
presentation
MoOPH is produced in a three-stage reaction sequence. First, the molybdenum (VI) oxide is oxidized to diperoxide and converted with hexamethylphosphoric triamide (HMPT) to form the hydrato complex. A drying step removes the water as ligands. The complex obtained in this way is reacted with pyridine to form MoOPH, which is obtained as a yellow powder.
literature
- Bang-Chi Chen, Ping Zhou, Franklin A. Davis, Engelbert Cianek: "α-Hydroxylation of Enolates and Silyl Enol Ethers", In: Organic Reaction , Wiley 2004 doi : 10.1002 / 0471264180.or062.01 .
Individual evidence
- ↑ a b Edwin Vedejs, S. Larsen: hydroxylation of enolate with Oxodiperoxymolybdenum (pyridines) (Hexamethylphosphoric triamides), MoO 5 · Py · HMPA (MoOPH): 3-hydroxy-1,7,7-trimethylbicyclo [2.2.1] heptane -2-one In: Organic Syntheses . 64, 1986, p. 127, doi : 10.15227 / orgsyn.064.0127 ; Coll. Vol. 7, 1990, p. 277 ( PDF ).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Dietrich Spitzner, Kai Oesterreich: "Anionically Induced Domino Reactions - Synthesis of a Norpatchoulenol-Type Terpene", in: European Journal of Organic Chemistry , 2001 , 10 , 1883–1886 doi : 10.1002 / 1099-0690 (200105) 2001: 10 <1883 :: AID-EJOC1883> 3.0.CO; 2-M
- ↑ JC Anderson, SC Smith: in Synlett 1990 , 107-108.
![{\ displaystyle \ mathrm {MoO_ {3} {\ xrightarrow [{2.HMPT}] {1.H_ {2} O_ {2}}} MoO_ {5} (H_ {2} O) [(Me_ {2} N) _ {3} PO]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5f51685e29576da508b2f3dd23b7ea3339dbaf7e)
![{\ displaystyle \ mathrm {{\ xrightarrow {0.2Torr}} MoO_ {5} [(Me_ {2} N) _ {3} PO]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c6aa416fbd401ad14ed89c91020610455282cd43)
![{\ displaystyle \ mathrm {{\ xrightarrow {pyridine}} MoO_ {5} [(Me_ {2} N) _ {3} PO] (pyridine)}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/30ea57401f1b8513c66c4e69d49f270ffd6339a2)