N , O -dimethylhydroxylamine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | N, O-dimethylhydroxylamine | |||||||||
| other names |
Methoxymethylamine |
|||||||||
| Molecular formula | CH 3 ONHCH 3 | |||||||||
| Brief description |
beige solid (hydrochloride) |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 61.08 g mol −1 | |||||||||
| Physical state |
|
|||||||||
| density |
0.89 g cm −3 |
|||||||||
| Melting point |
|
|||||||||
| boiling point |
42.4 ° C |
|||||||||
| Refractive index |
1.4152 (at 25 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
N , O -Dimethylhydroxylamine is a chemical compound from the group of amines . It is derived as a methyl group-substituted derivative of hydroxylamine .
Presentation and extraction
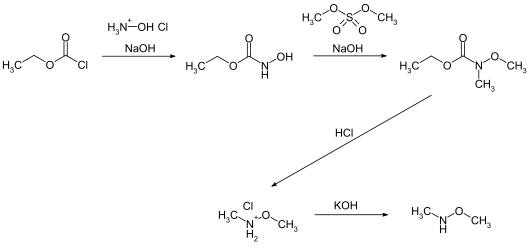
The synthesis of the compound starts with the reaction of ethyl chloroformate with hydroxylamine hydrochloride and subsequent methylation with dimethyl sulfate . The resulting intermediate compound gives the target compound as the hydrochloride by hydrolysis in the presence of concentrated hydrochloric acid . The free N , O- dimethylhydroxylamine can be obtained by reaction with potassium hydroxide solution and subsequent distillation.
use
N , O -dimethylhydroxylamine is used as a reagent for the production of Weinreb amides in the Weinreb amide ketone synthesis .
Individual evidence
- ↑ a b c d e data sheet N, O-dimethylhydroxylamine e, 98% from Sigma-Aldrich , accessed on March 3, 2015 ( PDF ).
- ↑ a b William M. Haynes: CRC Handbook of Chemistry and Physics, 95th Edition: . CRC Press, 2014, ISBN 978-1-4822-0867-2 , pp. 350 ( limited preview in Google Book Search).
- ^ A b Carl L. Yaws: The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals: Physical Properties for More Than 54,000 Organic and Inorganic Chemical Compounds, Coverage for C1 to C100 Organics and Ac to Zr Inorganics . Gulf Professional Publishing, 2015, ISBN 978-0-12-801146-1 , pp. 19 ( limited preview in Google Book search).
- ↑ a b c e-EROS Encyclopedia of Reagents for Organic Synthesis , 1999-2013, John Wiley and Sons, Inc., entry for N, O-Dimethylhydroxylamine, accessed October 16, 2015 .