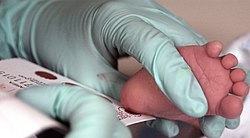
Newborn screening
Under newborn screening a nationally designed usually program for screening at one understands newborn . The aim is to test for certain congenital metabolic and hormonal diseases for which preventive treatment is possible and consequential damage can be avoided by starting treatment before the onset of the disease symptoms.
Basics
The aim and requirements of screening examinations are described in the article Screening . In Germany, a guideline of the Society for Neonatology and Pediatric Intensive Care Medicine, which was drawn up by a joint standing commission from pediatric and obstetric specialist societies, forms the basis for the content and organizational implementation of the newborn screening for metabolic disorders.
method
The sample is usually taken on the third day of life, in Germany often at the same time as the U2 examination . A deviation from the recommended collection period between 36 and 72 hours after the birth may require a check-up. For premature babies under the 32nd week of pregnancy, the screening is carried out twice, at the usual time period for mature newborns and again when the arithmetically reached 32 weeks of pregnancy.
By obtaining fewer drops of blood, usually from the heel, or alternatively from a vein, a filter paper card labeled with the patient data is completely and evenly saturated with blood in predetermined fields. The card is then dried for at least one hour at room temperature, but must never be heated for this. It will be sent to the screening laboratory the same day. Collection of samples over several days is not permitted. In addition to conventional test methods (determination of enzyme activity , colorimetric, immunological ), the blood sample is now also analyzed using tandem mass spectrometry .
Scope of the tests in Germany
According to the guidelines of the Federal Committee of Doctors and Health Insurance Funds on the early detection of illnesses in children up to the age of 6 ("Children's Guidelines"), the following are examined as part of the extended newborn screening:
- Hormonal diseases:
- Metabolic diseases:
- Biotinidase deficiency
- Galactosemia
- Phenylketonuria (PKU) and hyperphenylalaninemia (HPA)
- Maple Syrup Disease (MSUD)
- Medium Chain Acyl CoA Dehydrogenase Deficiency (MCAD)
- Long-chain-3-OH-acyl-CoA dehydrogenase deficiency (LCHAD)
- Very long chain acyl CoA dehydrogenase deficiency (VLCAD)
- Carnitine cycle defects:
- a) Carnitine palmitoyl transferase I deficiency (CPT-I)
- b) Carnitine palmitoyl transferase II deficiency (CPT-II)
- c) Carnitine acylcarnitine translocase deficiency (CACT)
- Glutaric aciduria type 1 (GA1)
- Isovaleric acidemia (IVA)
- Cystic fibrosis (cystic fibrosis)
- Type I tyrosinemia
Primary hypothyroidism and cystic fibrosis have the highest prevalence among the diseases examined.
- Early detection of immune deficiencies:
14. SCID (Severe combined Immunodeficiency), since August 2019
In addition to the extended newborn screening , a newborn hearing screening is carried out for the early detection of hearing disorders .
Scope of the tests in Austria
- Adrenogenital Syndrome
- Maple syrup disease
- Biotinidase deficiency
- Carnitine metabolic defects
- Galactosemia
- Type 1 glutaric aciduria
- Hypothyroidism
- Isovaleric acidemia
- LCHAD deficiency
- VLCAD deficiency
- MCAD deficiency
- Phenylketonuria
- Cystic fibrosis (cystic fibrosis)
Scope of tests in Switzerland
- Phenylketonuria
- Maple syrup disease
- Galactosemia
- Biotinidase deficiency
- MCAD deficiency
- Hypothyroidism
- Adrenogenital Syndrome
- Type 1 glutaric aciduria
- Cystic fibrosis, since 2011
- SCID (Severe combined immunodeficiency), since 2019, initially limited to 5 years
history
The history of newborn screening is closely linked to Robert Guthrie , who developed an easy-to-use test in newborns for phenylketonuria in 1962 . Horst Bickel had previously been able to prove in 1953 that early treatment can prevent the serious consequences of this disease. In the mid-1960s, the state of Massachusetts introduced the first newborn screening program.
In Germany, comprehensive screening for phenylketonuria was introduced in 1969/1970; over the years, other diseases were added and some were discarded. In 1997 the Permanent Screening Commission of the German Society for Pediatrics and Adolescent Medicine recommended screening for 5 diseases. Bavaria was one of the pioneers of expanded newborn screening in Germany with its newborn screening model project initiated in 1999, in which the participating children were tested for more than 100 congenital rare diseases. By means of new examination methods, the spectrum of diseases to be screened was significantly expanded in November 2002 compared to earlier screenings, even if it was much smaller than in the model project. The extended newborn screening with effect from April 1, 2005 and the newborn hearing screening with effect from January 1, 2009 were included in the children's guidelines as standard benefits for the first days of life for those with statutory health insurance. Since September 1, 2016, the screening has been expanded to include cystic fibrosis (CF) and since March 16, 2018, it has also included tyrosinemia.
literature
- S2k guidelines for newborn screening for congenital metabolic disorders and endocrinopathies of the Society for Neonatology and Pediatric Intensive Care Medicine (GNPI). In: AWMF online (as of 2011)
- Harms, Erik; Olgemöller, Bernhard: Newborn screening for metabolic diseases and endocrinopathies . In: Dtsch Arztebl Int . No. 108 (1-2) , 2011, pp. 11-22 ( Article ).
Web links
Individual evidence
- ^ German Society for Newborn Screening
- ↑ a b c Heidelberg University Hospital: Specialized Information Newborn Screening (PDF; 1.4 MB), accessed on November 20, 2019
- ↑ a b Guidelines of the Federal Committee of Doctors and Health Insurance Funds on the early detection of diseases in children up to the age of 6 (“Children's Guidelines”) , last amended on December 16, 2010 published in the Federal Gazette 2011; No. 40: p. 1013, entered into force on March 12, 2011
- ↑ DGNS eV - German Society for Newborn Screening eV Accessed on December 13, 2018 .
- ↑ detail. Retrieved February 17, 2020 .
- ^ Austrian program for the early detection of congenital metabolic diseases , accessed on October 14, 2012
- ↑ The Diseases To Look For (accessed April 22, 2014)
- ↑ Changes to the obligation to perform for medical services, resources and objects, analyzes and drugs as of January 1, 2019. BAG Bulletin 1 + 2/2019 , p. 13, accessed on February 19, 2019
- ↑ Jason Gonzales, Monte S. Willis: Robert Guthrie, MD, PhD published online in LabMedicine , accessed November 20, 2019
- ↑ Kristin Gatrell Bryant et al .: History of Newborn Screening in Medscape today available online , accessed August 6, 2011
- ↑ Gudrun Heyn, neonatal screening: A life-saving test , Pharmazeutische Zeitung (PZ) online, issue 23/2009
- ↑ Announcements: Resolution on an amendment to the guidelines of the Federal Committee of Doctors and Health Insurance Funds on the early detection of diseases in children up to the age of 6 (children guidelines) for the introduction of extended newborn screening of December 21, 2004 , announcement by the Federal Ministry for health and social security, published in Deutsches Ärzteblatt (Dtsch Arztebl 2005; 102 (16): A-1158 / B-970 / C-914, April 22, 2005), accessed on November 20, 2019
- ↑ Announcement of a resolution of the Federal Joint Committee on a change in the children's guidelines: Introduction of a newborn hearing screening , dated June 19, 2008, published by the German Society for Newborn Screening (DGNS eV), accessed on November 20, 2019
- ↑ Geraldine Nagel: Cystic fibrosis: newborn screening from September 1st, 2016. September 1, 2016, accessed December 14, 2016 .