Medium chain acyl CoA dehydrogenase deficiency
| Classification according to ICD-10 | |
|---|---|
| E71.3 | Fatty acid metabolism disorders |
| ICD-10 online (WHO version 2019) | |
Medium-chain acyl-coenzyme A dehydrogenase deficiency , also MCAD deficiency ( engl. Medium-chain acyl-CoA dehydrogenase deficiency (MCADD)), is one of the most common congenital metabolic disorders . It is a protein misfolding disease with loss of function in which the function of the enzyme medium chain acyl CoA dehydrogenase (MCAD) is impaired by a mutation . The defect in MCAD causes a reduced breakdown of medium-chain fatty acids. These fatty acids can therefore only be insufficiently used in the intermediate metabolism . The inheritance of MCAD deficiency is autosomal - recessive .
pathology
For energetic use in the cells , fatty acids are gradually shortened by removing small molecules by two carbon atoms. Under normal conditions, the fatty acids are converted into carbon dioxide and water , releasing energy . This process is called β-oxidation . A complex of seven enzymes is required to remove every two-unit. Since the process involves removing hydrogen atoms from an acyl group , the enzyme complex is called acyl dehydrogenase . The oxidation process of the fatty acids takes place within the mitochondria . Cytoplasmic fatty acids are bound to a carnitine molecule for transport across the mitochondrial membrane . The combination of carnitine and fatty acid is called acyl carnitine. MCAD-deficient patients have an increased concentration of medium-chain acyl-carnitines in the cytoplasm of the cells, as they can only insufficiently break down the acyl-carnitines. The medium-chain fatty acids consist of 6 to 12 carbon atoms. The medium-chain acyl carnitines are partly secreted by the cells and thus enter the bloodstream.
Patients with decreased MCAD activity have a fatty acid oxidation disorder. In normal health, this does not lead to any impairment. However, the disorder can lead to hypoglycemia (low blood sugar), hyperammonaemia and possibly sudden death in the case of another illness that causes a longer fasting period . If the MCAD deficiency is discovered at an early stage, a metabolic imbalance can be counteracted very safely in these situations by adequate food intake and, if necessary, by glucose infusions in the hospital. While until a few years ago an undiscovered MCAD deficiency led to death in around 25% of cases the first time a metabolic imbalance occurred, the occurrence of such a metabolic crisis can be avoided in almost all cases due to the early detection of an MCAD deficiency prevent very effectively.
Examination for Sudden Infant Death
In a few cases (approx. 1%) of sudden infant death , an MCAD deficiency was found retrospectively. Due to this rarity, a causal involvement is suspected, but there is no definite proof of the MCAD deficiency as the actual cause of death, especially with regard to sudden infant death.
When examining a child who has died suddenly as an infant, blood samples are taken to determine the concentration of acyl-carnitine in the blood. An MCAD deficiency can be concluded when the acyl carnitine level in the blood has risen to a typical value.
If the time between sudden infant death and the autopsy is not too long, it is sometimes possible to take fibroblasts from the dermis from a skin sample during the examination. Fatty acids labeled with radioactive carbon atoms (C-14) can be added to the cultivated medium. When the cells oxidize and absorb these fatty acids within their metabolism, radioactive carbon dioxide is generated, which can be detected with a suitable device.
The production rate of carbon dioxide due to fatty acid chains of different lengths can be used as a test to determine whether there is a deficiency in one of the acyl dehydrogenases. This test can be used to support the diagnosis of MCAD deficiency if the suspicion is based on the pattern of the acyl carnitines.
genetics
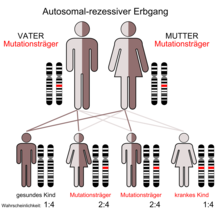
Mutations in the ACADM gene lead to a misfolding of the MCAD enzyme. In humans, the gene is located on chromosome 1 gene locus p31. The misfolded enzymes are sorted out by the protein quality control and broken down in the proteasome . The affected patients are therefore deficient in medium chain acyl-CoA dehydrogenase. The condition is inherited as an autosomal recessive trait. This means that two copies of a faulty gene are necessary in each cell to cause the deficiency. If only one copy is defective, then the person is an asymptomatic carrier and will not develop MCAD deficiency. The frequency of MCAD deficiency is assumed to be around 1 in 10,000.
diagnosis
The medium-chain acyl carnitines can be detected in the plasma, for example, by means of tandem mass spectrometry . This can be done as part of a newborn screening . An increased concentration of activated fatty acids of medium chain length (6–12 carbon units) within the acyl carnitine suggests a MCAD deficiency. The concentration of octanoylcarnitine serves as the key parameter. A suspicious initial finding is usually checked by a control screening and, if necessary, by a molecular genetic examination ( DNA analysis ).
The clinical suspicion of MCAD deficiency can be corroborated by a blood count if hypoglycaemia , metabolic acidosis and hyperuricaemia are diagnosed at the same time .
variants
Before the introduction of the extended newborn screening, the MCAD deficiency was in most cases only determined in the examinations after an acute metabolic imbalance. In around 80% of the cases, the mutation K329E (other name c.985A> G; a point mutation ) became homozygous, i.e. H. found on both copies of the gene. In another 18% of the cases, this genetic defect was involved in connection with another mutation ( compound heterozygous ). For this reason, K329E is classified as a high risk variant worldwide.
In the molecular genetic examinations after suspicious screening findings, however, a whole series of other mutation combinations have now been found that have never been clinically evident before. It is therefore believed that in most of these cases it is a mild MCAD deficiency phenotype . Patients with these mild variants will probably remain asymptomatic for their entire life even without treatment, but conclusive evidence can only be obtained with an appropriately extended fasting test (> 24 hours). Since such a test would be associated with the increased risk of a metabolic imbalance, both patients with the high-risk variant and those with a likely mild MCAD deficiency expression in the metabolic centers are usually treated in the same way (e.g. with glucose infusions in the event of illness ) treated.
Initial description
Medium-chain acyl-CoA dehydrogenase deficiency was first described in 1976 by the Danish molecular geneticist Niels Gregersen and colleagues.
Individual evidence
- ↑ LMU Munich: Protein misfolding.
- ^ EM Maier, SW Gersting et al .: Protein misfolding is the molecular mechanism underlying MCADD identified in newborn screening. In: Human molecular genetics . Volume 18, Number 9, May 2009, pp. 1612-1623, ISSN 1460-2083 . doi: 10.1093 / hmg / ddp079 . PMID 19224950 . PMC 2667288 (free full text).
- ↑ a b c d I. Knerr, U. Nennstiel-Ratzel u. a .: Medium chain acyl CoA dehydrogenase deficiency: a clinically important metabolic disorder. In: Dtsch Arztebl. Volume 102, 2005, pp. A-2565 / B-2166 / C-2045.
- ^ GF Hoffmann, R. von Kries et al .: Frequencies of inherited organic acidurias and disorders of mitochondrial fatty acid transport and oxidation in Germany. In: European Journal of Pediatrics. Volume 163, Number 2, February 2004, pp. 76-80, ISSN 0340-6199 . doi: 10.1007 / s00431-003-1246-3 . PMID 14714182 .
- ↑ A. Schulze, M. Lindner u. A .: Expanded newborn screening for inborn errors of metabolism by electrospray ionization-tandem mass spectrometry: results, outcome, and implications. In: Pediatrics. Volume 111, Number 6 Pt 1, June 2003, pp. 1399-1406, ISSN 1098-4275 . PMID 12777559 .
- ↑ Hyperuricemia as the main symptom in the acyl-coenzyme A dehydrogenase defect of medium-chain fatty acids. In: Klin Padiatr. 1997; 209 (6), pp. 357-360, doi: 10.1055 / s-2008-1043975 .
- ↑ Medium-chain acyl-CoA dehydrogenase deficiency: a clinically important metabolic disorder. In: Dtsch Arztebl. 2005; 102 (38), pp. A-2565 / B-2166 / C-2045.
- ↑ N. Gregersen, R. Lauritzen, K. Rasmussen: Suberylglycine excretion in the urine from a patient with dicarboxylic aciduria. In: Clinica chimica acta. Volume 70, Number 3, August 1976, pp. 417-425, ISSN 0009-8981 . PMID 947635 .
Web links
- Medium chain acyl CoA dehydrogenase deficiency. In: Online Mendelian Inheritance in Man . (English)
- Medium chain acyl CoA dehydrogenase deficiency. In: Orphanet (Rare Disease Database).
- Medium chain acyl-CoA dehydrogenase deficiency newbornscreening.info (English)
- ACADM gene (US National Library of Medicine)