Oliceridine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
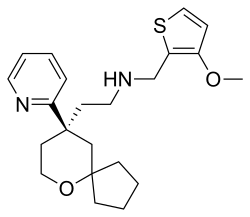
|
||||||||||
| General | ||||||||||
| Non-proprietary name | Oliceridine | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 22 H 30 N 2 O 2 S | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 386.55 g mol −1 | |||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Oliceridine is a drug used to treat acute pain that is so severe that intravenous administration of an opioid analgesic is required and alternative treatments are inadequate.
properties
Oliceridin is a functionally selective agonist on the μ- opioid receptor . It exerts its effects via activation of the G protein - signal path and has both an the morphine -like power and efficiency. It avoids the action on β-arrestin 2 and thus the receptor internalization . This strategy aims to reduce the side effects typical of conventional opioids such as respiratory depression , constipation and tolerance .
Oliceridin has a chiral carbon atom and thus an enantiomeric variant, the ( S ) -TRV130. This has a pharmacological spectrum of activity similar to that of the ( R ) form (= oliceridine), but 90 times less effective. This difference is attributed to a significantly lower rate of association at the receptor.
The pharmaceutically used salt of fumaric acid , oliceridine fumarate , is a white to slightly colored solid that is sparingly soluble in water. Another salt is oliceridine hydrochloride , a white solid that is practically insoluble in water.
development
The development of olceridine is based on the strategy of " biased agonism ". This means that biologically active substances, after binding to a receptor, preferentially activate one of several possible signal transduction pathways (functional selectivity). The US Food and Drug Administration (FDA) granted olceridine Break Through Therapy status in February 2016 . After the FDA initially rejected an application for drug approval in 2018 due to insufficient information on application safety, it granted approval (trade name Olinvyk ) in August 2020 after the application was revised by the pharmaceutical company Trevena Inc.
Individual evidence
- ↑ a b c medkoo.com: MSDS-TRV130.pdf , accessed on June 26, 2016.
- ↑ DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD: AG protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine . In: Journal of Pharmacology and Experimental Therapeutics . 344, No. 3, March 2013, pp. 708-17. doi : 10.1124 / jpet.112.201616 . PMID 23300227 .
- ↑ MF Pedersen, TM Wróbel, E. Märcher-Rørsteda, DS Pedersen, TC Møller, F. Gabrielea, H. Pedersen, D. Matosiukc, SR Foster, M. Bouvier, H. Bräuner-Osborne: Biased agonism of clinically approved μ -opioid receptor agonists and TRV130 is not controlled by binding and signaling kinetics . In: Neuropharmacology . tape 166 , April 2020, doi : 10.1016 / j.neuropharm.2019.107718 .
- ↑ Olyvik, label . As of August 2020 ( PDF )
- ↑ External identifiers from or database links to Oliceridinhydrochlorid : CAS Number: 1401031-39-7, PubChem : 68313941 , Wikidata : Q98273156 . Molar mass: 423.01 g mol −1 .
- ↑ H. Blasius: Which novel opioids are in the pipeline? , Deutsche Apothekerzeitung, April 24, 2018.
- ↑ Trevena, Inc. Receives FDA Breakthrough Therapy Designation for Oliceridine for the Management of Moderate-to-Severe Acute Pain , businesswire.com, February 22, 2016.
- ↑ FDA rejects Trevena's painkiller oliceridine | FierceBiotech ( en ) Questex LLC. Retrieved December 23, 2018.