Patient-directed analgesia
The patient-controlled analgesia ( english patient-controlled analgesia , PCA), also patient controlled or patient-controlled pain therapy called, allows the patient to a pain reliever ( analgesic to administer) upon the occurrence of pain itself. The concept of PCA and the first prototypes of PCA pumps were developed in 1971 by Philip H. Sechzer.
Forms of PCA
Orally
Over-the-counter or prescription medication given by mouth (as tablets or capsules) is the most common form of PCA.
Intravenous, subcutaneous
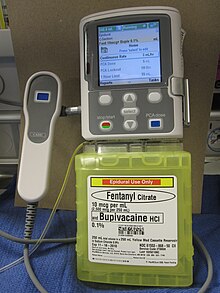
The intravenous or subcutaneous administration (colloquially called "pain pump" hereinafter) is a concept of pain therapy , the post-operatively or in the palliative care is used. While the term is not primarily limited to a form of application or a group of drugs, an opioid solution is administered intravenously or subcutaneously by activating a microprocessor-controlled infusion pump or an appropriately designed elastomer pump (PCA pump). After the self-administration of a defined dose of the drug, a lock is activated, which enables a new injection only after a set time window. In addition to the exclusively intermittent administration of painkiller boluses, there is also the option of permanently injecting a basic requirement. PCA is a safe procedure; respiratory depression is rarely a side effect of pain relievers.
The use should enable the patient to be independent and ensure that the dose is optimally adapted to the needs. The effectiveness of pain management is generally better than that of intermittent administration by medical personnel.
Epidural
Alternatives to intravenous administration of analgesics are administration via an epidural catheter ( patient-controlled epidural analgesia , PCEA) or a peripheral pain catheter ( patient-controlled regional analgesia , PCRA).
nasal
When painkillers are administered nasally (as a nasal spray), they are absorbed through the nasal mucous membranes. Compared to the oral intake, the bioavailability of the active ingredient is higher.
inhalation
With oral inhalation using a special inhaler , the pain reliever is absorbed through the lungs.
As early as 1968, Robert Wexler, Abbott Laboratories, registered a patent for the Analgizer , an inhaler for administering methoxyflurane to treat pain. While intranasal administration has a bioavailability of 40%, it is significantly improved with oral inhalation, as was shown in a randomized double-blind phase III study based on dihydroergotamine for the treatment of migraines .
Transdermal
When administered transdermally , for example as a patch , the agent is absorbed through the skin.
Individual evidence
- ↑ PH Sechzer: Studies in pain with the analgesic-demand system . In: Anesthesia and Analgesia . tape 50 , no. 1 , February 1971, p. 1-10 , PMID 5100236 .
- ↑ J. Hense, M. Przyborek, J. Rosenbruch, C. Ostgathe, C. Wolf, S. Bogner: SOP - Subcutaneous drug administration and infusions in adult palliative medicine. The Oncologist, June 23, 2017, DOI10.1007 / s00761-017-0247-1; accessed on December 10, 2019
- ↑ a b Jeffrey A. Grass: Patient-controlled analgesia . In: Anesthesia and Analgesia . tape 101 , 5 Suppl, November 2005, pp. S44-61 , PMID 16334492 .
- ↑ B. Walder, M. Schafer, I. Henzi, MR Tramèr: Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review . In: Acta Anesthesiologica Scandinavica . tape 45 , no. 7 , August 2001, p. 795-804 , PMID 11472277 .
- ↑ JC Ballantyne, DB Carr, TC Chalmers, KB Dear, IF Angelillo, F. Mosteller: Postoperative patient-controlled analgesia: meta-analyzes of initial randomized control trials . In: Journal of Clinical Anesthesia . tape 5 , no. 3 , June 1993, p. 182-193 , PMID 8318237 .
- ↑ Analgizer , accessed on August 20, 2014
- ↑ Migraine Therapy , accessed August 20, 2014
Web links
- www.zwai.net - Patient- controlled analgesia - Practical and nursing aspects of PCA in everyday clinical practice