Pechmann pyrazole synthesis
The Pechmann pyrazole synthesis is a name reaction of organic chemistry , which was discovered in its classical method by the German chemist Hans von Pechmann (1850–1902). Pyrazoles were synthesized by Ludwig Knorr as early as 1883 .
Overview reaction
The slow reaction between diazomethane and acetylene gives the product pyrazole :
Reaction mechanism
Diazomethane occurs in three different mesomeric boundary structures :
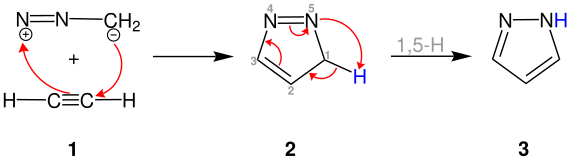
The following shows the Pechmann pyrazole synthesis in its classic variant, the reaction between diazomethane and acetylene 1 . First, the carbon atom of acetylene 1 is attacked by the negatively charged carbon atom of diazomethane. The non-aromatic 3 H -pyrazole 2 is formed by this 1,3-dipolar cycloaddition .
The product pyrazole 3 is formed by the subsequent 1,5 H shift . The reaction can also be carried out with alkenes . When using ethylene , for example, pyrazoline is obtained. However, there is no further H shift.
In the next example, the second mesomeric boundary structure of the diazomethane drawn in the example above is reacted with the ester 4 . Here, too, a dipolar 1,3-cycloaddition takes place, whereby the pyrazoline derivative 5 is formed.
A 1,3-H shift now occurs with the formation of structure 6 . The pyrazole derivative 7 can be obtained by oxidation .
disadvantage
The method used by Pechmann required the use of carcinogenic hydrazines. In addition, problems with the regioselectivity of the reaction were common, which resulted in limited substrate scope. That is why an alternative is presented below.
Modern variant
In 2010 Neumann, Suri and Glorius described a modern method for the synthesis of tetrasubstituted pyrazole derivatives from esters of enamines and nitriles . The above-mentioned disadvantages of older synthetic routes have been almost completely eliminated. The following mechanism was proposed:
First, the Lewis acidic copper nitrile 8 coordinates to the enamino ester 9 . A binding electron pair of the C = C double bond of the nucleophilic enamino ester forms a bond with the partially positively polarized carbon atom of the nitrile. The 1,3-bisimine 10 is formed by eliminating an acetic acid molecule . In this variant, the targeted determination of the residues makes it possible to form unsymmetrical pyrazoles with almost complete regioselectivity.
The copper (II) chelate complex 11 is formed through renewed elimination of an acetic acid molecule and oxidative NN bond formation. Subsequent reductive elimination of copper yields the tetrasubstituted pyrazole derivative 12 .
literature
- Jie Jack Li: Name reactions, a collection of detailed reaction mechanism . Vol 1. Springer 2002. ISBN 3-540-43024-5 .
- Z. Wang: Comprehensive Organic Name Reactions and Reagents , Vol 2. John Wiley & Sons, Hoboken, New Jersey 2009, pp. 2147-2150, ISBN 978-0-471-70450-8 .
- Theophil Eichner, Siegfried Hauptmann, Andreas Speicher: The chemistry of heterocycles: structure, reactions, synthesis, and applications , Vol 3. Wiley-VCH, Weinheim 2012, pp. 236-243, ISBN 978-3-527-32868-0 .
- Paul Walden: History of Organic Chemistry since 1880 , Vol. 2. Springer-Verlag, Berlin 1972.
- Hermann Römpp, Jürgen Falbe, Eckard Amelingmeier: Römpp-Lexikon Chemie , Vol 10. Thieme-Verlag, Stuttgart 1999, p. 2183, ISBN 3-13-107830-8 .
- Robert Ebermann, Ibrahim Elmadfa: Textbook Food Chemistry and Nutrition , Vol 1. Springer-Verlag, Vienna 2011, p. 382, ISBN 978-3-7091-0210-7 .
Individual evidence
- ↑ Ludwig Knorr: Effect of acetoacetic ester on phenylhydrazine , reports of the German chemical society 1883, 2 , 2597-2599, doi: 10.1002 / cber.188301602194 .
- ↑ H. v. Pechmann: Pyrazole from acetylene and diazomethane , reports of the German chemical society 1898, 3 , 2950–2951, doi: 10.1002 / cber.18980310363 .
- ↑ Julia J. Neumann, Mamta Suri, Frank Glorius: Efficient pyrazole by oxidative CC / NN-Bindungsknüpfungskaskade , Angewandte Chemie 2010, 42 , 7957-7961, doi: 10.1002 / ange.201002389 .