Peramivir
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
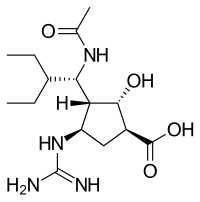
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Peramivir | |||||||||||||||
| other names |
(1 S , 2 S , 3 R , 4 R ) -3 - [( S ) -1-Acetamido-2-ethylbutyl] -4-guanidino-2-hydroxycyclopentanecarboxylic acid ( IUPAC ) |
|||||||||||||||
| Molecular formula | C 15 H 28 N 4 O 4 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action |
Inhibition of viral neuraminidase |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 328.41 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Peramivir is an experimental drug from the group of neuraminidase inhibitors that is being developed for the treatment of influenza . Peramivir has a virostatic effect by inhibiting the viral neuraminidase , an enzyme that enables the release of the newly formed viruses from the host cell.
Peramivir differs from the neuraminidase inhibitors zanamivir and oseltamivir in that it is given intravenously.
In October 2009, the US Food and Drug Administration (FDA) approved peramivir as a solution for injection for the treatment of the 2009 pandemic influenza H1N1 ("swine flu") in emergencies. The drug is not approved for the treatment of seasonal influenza or uncomplicated H1N1 diseases. The FDA's peramivir information for physicians, with a call to report observed adverse events to the FDA within seven days, has been released. This emergency approval was revoked on June 23, 2010. Peramivir can be imported from Japan, where it is approved under the finished drug name Rapiacta .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Debra Birnkrant, Edward Cox: The Emergency Use Authorization of Peramivir for Treatment of 2009 H1N1 Influenza. In: New England Journal of Medicine. 361, 2009, p. 2204, doi : 10.1056 / NEJMp0910479 .
- ↑ Peramivir EUA, Fact Sheet for HCP Authorized by FDA on October 23, 2009 ( en , PDF; 391 kB) p. 42. Retrieved on November 4, 2009.
- ↑ Peramivir Emergency Use Authorization Disposition Letter and Question and Answer Attachment
- ↑ Shionogi Launches Rapiacta In Japan - Pharmaceutical Business Review