Perillaaldehyde
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
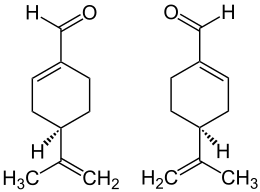
|
||||||||||
| Structural formulas of the ( R ) -form (left) and the ( S ) -form (right) | ||||||||||
| General | ||||||||||
| Surname | Perillaaldehyde | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 10 H 14 O | |||||||||
| Brief description |
yellow liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 150.22 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
|
|||||||||
| boiling point |
|
|||||||||
| Refractive index |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
Perillaaldehyde is a chemical compound from the group of aldehydes that occurs in two enantiomeric forms. It belongs to the natural product class of monoterpenes .
Occurrence
Perillaaldehyde occurs naturally in perilla oil and cumin . Both enantiomeric forms generally occur in nature.
properties
( S ) - (-) - Perillaaldehyde is a yellow oily liquid.
use
( S ) - (-) - Perillaaldehyde is used as a starting product for the production of nature-identical substances. Its oxime perillartin (perilla sugar) is 2000 times sweeter than sugar and is used as a sugar substitute in Japan.
toxicology
According to EFSA and the latest evaluations of a study, perillaaldehyde causes DNA damage to the liver. However, "some weaknesses in the historical control data" of the laboratory conducting the studies were also identified.
Individual evidence
- ↑ a b c d e f g data sheet (S) - (-) - Perillaldehyde, ≥92% from Sigma-Aldrich , accessed on December 3, 2013 ( PDF ).
- ↑ a b c d e Ashutosh Kar: Pharmacognosy And Pharmacobiotechnology . New Age International, 2003, ISBN 81-224-1501-6 , pp. 321 ( limited preview in Google Book search).
- ↑ He-ci Yu, Kenichi Kosuna, Megumi Haga: Perilla: The Genus Perilla . CRC Press, 1997, ISBN 1-4398-2271-9 , pp. 129 ( limited preview in Google Book search).
- ↑ Robert Ebermann, I. Elmadfa: Textbook food chemistry and nutrition . Springer DE, 2008, ISBN 3-211-49348-4 , pp. 262 ( limited preview in Google Book search).
- ↑ a b from Atta-ur-Rahman: Stereoselective Synthesis (Part J) - Atta-ur-Rahman . Elsevier, 1995, ISBN 0-08-054178-X , pp. 230, 251 ( limited preview in Google Book search).
- ↑ EFSA: Flavoring substance considered a safety concern

