Parylene
As Parylene a group are inert , hydrophobic , optically transparent , polymeric coating materials designated with a wide range of industrial applications. In addition to the hydrocarbon poly- p- xylylene (often referred to as parylene N ), halogenated polymers are also used.
The coating is applied in a vacuum by resublimation from the gas phase as a pore-free and transparent polymer film on the substrate . Practically every substrate material such as B. Metal , glass , paper , paint , plastic , ceramic , ferrite and siliconecoatable with parylenes. Due to the gaseous deposition, areas and structures can also be reached and coated that cannot be coated with liquid-based methods, such as B. sharp edges and points or narrow and deep crevices. Coating thicknesses of 0.1 to 50 µm can be applied in one operation.
properties
| Parylene | |||||
| Structural formula |

|
||||
| Substituents | X | R 1 | R 2 | R 3 | R 4 |
| Parylene N | H | H | H | H | H |
| Parylene C. | H | Cl | H | H | H |
| Parylene D | H | Cl | H | Cl | H |
| Parylene HT | F. | H | H | H | H |
Parylenes are hydrophobic , chemically resistant plastics with good barrier properties against inorganic and organic media, strong acids , alkalis , gases and water vapor. As a thin and transparent coating with high gap clearance, they are suitable for substrates with a complex design. Since parylene has no liquid phase, no edge alignment is formed. Parylenes have good dielectric properties with high dielectric strength and low dielectric constant . As a biostable and biocompatible coating, parylene have an FDA master file. From a layer thickness of 0.6 µm they are free of micropores and pinholes. The coating takes place without thermal stress on the substrates at room temperature in a vacuum . This process offers a very high level of corrosion protection and a uniform layer formation, which - depending on the parylene type - is temperature-stable up to 350 ° C (Parylene HT), mechanically stable and produces low mechanical stress, conditionally (Shore R80-R120; depending on the type ) is abrasion-resistant and does not cause outgassing. This surface can be activated by low pressure plasma or other adhesion promoters.
Manufacturing
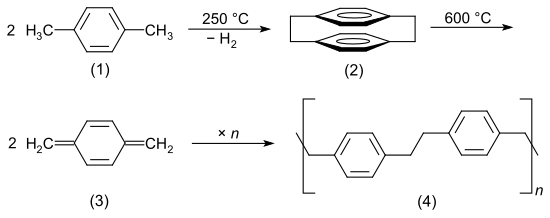
Parylenes are produced by chemical vapor deposition . The starting material is p- xylene (1) (or halogenated derivatives ). This is evaporated and passed through a high temperature zone. A reactive [2,2] -paracyclophane (2) is formed, which breaks down to 1,4-quinonedimethane (3). The quinone dimethane polymerizes immediately on surfaces to form chain-like poly- p- xylylene (4).
Typical applications
Typical uses for parylene are:
- conformal coating of electronic components for harsh environmental conditions (meets "MIL-I-46058C, Type XY" (this standard was set to "do not use for new designs" on November 30, 1998))
- Electronics for the military (extension of shelf life, protection against environmental influences and operational contamination, e.g. salt water, oil, fuel)
- Electronics for space travel (extension of shelf life)
- dielectric coating (e.g. cores / coils)
- hydrophobic coating (protection from moisture)
- Barrier layers (e.g. for filters, membranes, valves)
- Corrosion protection for metallic surfaces
- Reinforcement of microstructures
- Abrasion protection
- Protection of plastic, rubber, etc. from harmful environmental influences
- Reduction of friction (e.g. with guide wires for catheters)
- biocompatible coating of medical implants (e.g. transponders for animal registration, biomedical tubes, heart implants)
Individual evidence
- ^ Arnold Willmes, Pocket Book Chemical Substances , Harri Deutsch, Frankfurt (M.), 2007.
Web links
- Specialty Coating Systems: Deposition Process
- Specialty Coating Systems: Properties