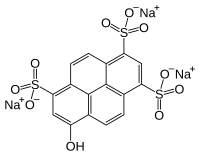
Pyranine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Pyranine | |||||||||||||||
| other names | ||||||||||||||||
| Molecular formula | C 16 H 7 Na 3 O 10 S 3 | |||||||||||||||
| Brief description |
yellow powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 524.39 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.15 g cm −3 |
|||||||||||||||
| Melting point |
> 300 ° C (decomposition) |
|||||||||||||||
| solubility |
slightly soluble in water (178 g l −1 ) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Pyranine is a fluorescent dye in the pyrene dye family .
properties
Pyranine is a yellow solid that is easily soluble in water. The solution shows an intense yellow-green fluorescence, the intensity of which depends on the pH value .
use
As a fluorescent dye , pyranine is used, among other things, as a hair dye , in soaps and in highlighters . It is also used to color detergents and as a marking fluid for water circuits. It is also approved for marking experiments on bodies of water. The detection limit in a commercially available fluorescence spectrometer is around 5 · 10 −8 g · l −1 , the emission maximum at 512 nm.
The dependence of fluorescence on the pH value can be used in microbiology to determine the intracellular pH value.
further reading
- A. Herbst, HJ Wygoda: Pyranin - a fluorescent dye for application-technical experiments. (PDF; 620 kB) In: Nachrichtenbl Deut Pflanzenschutzd. Volume 58, Number 3, 2006, pp. 79-85.
- JV Thomas, MR Brimijoin et al: The fluorescent indicator pyranine is suitable for measuring stromal and cameral pH in vivo. In: Experimental eye research. Volume 50, Number 3, March 1990, pp. 241-249, PMID 2156724 .
- OF Mohammed, J. Dreyer a. a .: Solvent-dependent photoacidity state of pyranine monitored by transient mid-infrared spectroscopy. In: Chemphyschem: a European journal of chemical physics and physical chemistry. Volume 6, Number 4, April 2005, pp. 625-636, doi: 10.1002 / cphc.200400510 . PMID 15881578 .
Individual evidence
- ↑ Datasheet Pyranin 120% at Lanxess, as of January 2011.
- ↑ Data sheet 8-Hydroxy-1,3,6-pyrenetrisulfonic acid tri sodium salt at Acros, accessed on January 1, 2012.
- ↑ a b c Data sheet 8-Hydroxypyrene-1,3,6-trisulfonic acid trisodium salt from Sigma-Aldrich , accessed on January 1, 2012 ( PDF ).
- ↑ a b c Bavarian State Office for Water Management: Instructions for the implementation and assessment of marking experiments in bodies of water. (PDF; 334 kB) Leaflet No. 3.1 / 1, as of June 6, 2002.
- ↑ Y. Avnir, Y. Barenholz: pH determination by pyranine: medium-related artifacts and Their correction. In: Analytical biochemistry . Volume 347, number 1, December 2005, pp. 34-41, doi: 10.1016 / j.ab.2005.09.026 . PMID 16289011 .
- ↑ M. Bährle-Rapp: Springer Lexicon cosmetics and body care. Edition 2, Verlag Springer, 2004, ISBN 3-540-20416-4 , p. 417 ( limited preview in the Google book search).
- ↑ a b Aqueous dyes ( Memento from January 27, 2013 in the web archive archive.today ). lanxess.com, accessed December 31, 2011.
- ↑ BS Gan, E. Krump et al .: Loading pyranine via purinergic receptors or hypotonic stress for measurement of cytosolic pH by imaging. In: American Journal of Physiology-Cell Physiology . 1998; 275: p. C1158-C1166, PMID 9755070 .
- ↑ K. Kano, JH Fendler: Pyranine as a sensitive pH probe for liposome interiors and surfaces. pH gradients across phospholipid vesicles. In: Biochimica et biophysica acta . 1978; 509: p. 289-299, PMID 26400 .
- ↑ S. Zhang, S. Tanaka et al: Fiber-optical sensor based on fluorescent indicator for monitoring physiological pH values. In: Medical & biological engineering & computing. 1995; 33: p. 152-156, PMID 7643652 .
