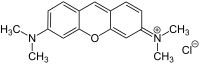
Pyronine G.
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Pyronine G. | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 17 H 19 ClN 2 O | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 302.80 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
250-260 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Pyronin G (also Pyronin Y) is a dye from the group of Pyronine . It is a cationic xanthene dye .
Surname
The G is an abbreviation for yellow, so that the substance in the English-speaking world contains a Y for yellow. Yellow describes the absorption maximum of the substance in the yellow-green area of the light spectrum .
description
The quaternary nitrogen is methylated. If there are ethyl groups instead, the substance is called pyronine B (bluish):
Like pyronine G, it is cationic.
Manufacturing
Pyronine G can be synthesized by condensation of N , N -dimethyl- m -aminophenol with formaldehyde to give tetramethyldiaminodioxydiphenylmethane, which cyclizes to tetramethyldiaminoxanthene by means of sulfuric acid with elimination of water .
use
For blood tests, Artur Pappenheim introduced the fabric for dyeing purposes in 1899, which became known as Pappenheim dye . The method was modified in 1902 by Paul Gerson Unna for tissue studies so that it was called Pappenheim-Unna staining.
Histology uses the substance in combination with methyl green to stain nucleic acids in the specimen. In this intercalated pyronine G with RNA while Methyl Green binds to DNA. In flow cytometry, it is therefore an alternative to acridine orange for differential diagnosis .
The binding behavior depends on the dye concentration as well as on the conformation of the nucleic acids, which in turn varies depending on the phase of mitosis . The toxic effect on live samples is due to the binding.
Derivatives that are formed by reacting with cyanides to form nitrile at the 9'-C with subsequent oxidation are used as textile dyes.
literature
- FWD Rost: Fluorescence microscopy , 1995, p. 357 ( limited preview in the Google book search).
- Howard Maurice Shapiro: Practical flow cytometry , 2003, pp. 323-324 ( limited preview in Google book search).
- James Sheridan Muspratt, Ernst Otto Beckmann, Hans Bunte, Bernhard Neumann, Arthur Heinrich Binz, Fritz Hayduch, Friedrich Karl Adolf Stohmann: Encyklopädisches Handbuch der technischen Chemie , Volume 3, Part 1, 1915, p. 448.
- Fritz Ullmann, Matthias Bohnet: Ullmann's encyclopedia of industrial chemistry , Volume 37, 2003, p. 479.
Web links
- AppliChem: Pyronin Y (CI 45005)
- ihcworld.com: ABC of Safety in the Biological Sciences - Pyronin G
Individual evidence
- ↑ a b c d sheet pyronine Y, for microscopy (Bot., Fl., Hist.) At Sigma-Aldrich , accessed 30 January 2012 ( PDF ).
