Rubottom Oxidation
The Rubottom oxidation (also called Rubottom reaction ) is a name reaction in organic chemistry in which a ketone or aldehyde is converted into the corresponding α-hydroxy ketone or aldehyde .
It was discovered in 1974 by several research groups independently of one another and described in the literature: The first publication of this reaction goes back to the Canadian chemist Adrian G. Brook (1924-2013) on June 10th. Then the reaction was described by a group led by the American chemist Alfred Hassner (* 1930) on September 4th. On September 24th, the reaction was finally submitted by a group led by the American chemist George M. Rubottom (* 1940), after whom the reaction was ultimately named.
Overview reaction
In the Rubottom reaction, a ketone or aldehyde on the α-carbon atom is oxidized with meta -chloroperbenzoic acid ( m -CPBA) to give the corresponding α-hydroxy compound. The reaction for acetone is shown as an example :
Reaction mechanism
A possible reaction mechanism is presented using the example of acetone.
In the first step, a silyl enol ether ( 2 ) is produced by reacting acetone ( 1 ) with triethylsilyl triflate and triethylamine ( variant (a) ) or with lithium diisopropylamide (LDA) and chlor (trimethyl) silane (TMS-Cl) ( variant (b) ):
- In variant (a) , acetone ( 1 ) first reacts with the hard electrophile triethylsilyl triflate on the carbonyl oxygen atom to form cation ( 1a ), which is then deprotonated by the weak base triethylamine , so that the silyl enol ether ( 2 ) is formed.
- In variant (b) the reaction path is slightly different: Here acetone ( 1 ) reacts with LDA to form the lithium enolate ( 1b ), which reacts with TMSCl to form the silyl enol ether ( 2 ).
In the second step, the corresponding α-hydroxy compound is finally formed from the silyl enol ether. It comes with the addition of z. B. m -CPBA to the enol ( 2 ) for epoxidation , so that the siloxyepoxide ( 3 ) is formed. The epoxy ring opens under acidic conditions and a carbocation ( 4 ) is formed, which is stabilized by anomeric effects . The Brook rearrangement (1,4-silyl migration) produces the α-siloxy ketone ( 5 ), in which only the silyl group has to be removed in order to obtain the desired α-hydroxy compound ( 6 ) . This can be done by adding tetrabutylammonium fluoride (TBAF) and then acidic processing. Since only the fluoride ion of TBAF is involved in the reaction, only this is shown in the figure for the reaction mechanism.
Atomic economy
The Rubottom oxidation has established itself as a method to produce α-hydroxy carbonyl compounds, but is not necessarily recommendable because of its atom economy . On the one hand, stoichiometric amounts of a trialkylsilyl halide , a base and m -CPBA are used and, on the other hand, large amounts of waste are produced. For the latter reason in particular, alternatives are being sought to produce α-hydroxy carbonyl compounds. These include B. the use of dimethyldioxirane (DMDO) or oxygen from the air as an oxidizing agent .
Application examples
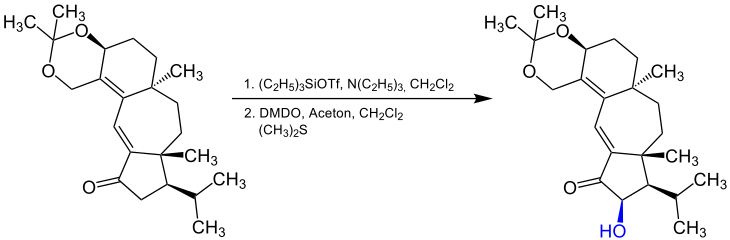
It is possible to carry out the α- hydroxylation enantioselectively under certain conditions . One example of this is the synthesis of guanakastepene A , which goes back to the American chemist Samuel Danishefsky (* 1963) and colleagues. This reaction also shows that the Rubottom oxidation can be carried out with DMDO:
Another example of the application of Rubottom Oxidation can be found in the synthesis of FR901464, a chemical compound that acts against tumors . This synthesis goes back to a group led by the American chemist Eric N. Jacobsen (* 1960). In the production of the substance, the Rubottom oxidation is a partial step in order to synthesize an important partial compound of FR901464. A silyl enol ether undergoes the Rubottom reaction under modified conditions ( buffer and non-polar solvent ).
Individual evidence
- ↑ a b c L. Kürti, B. Czakó: Strategic Applications of Named Reactions in Organic Synthesis. Elsevier Academic Press, Burlington / San Diego / London 2005, ISBN 0-12-369483-3 , pp. 388-389.
- ^ A b c Z. Wang: Comprehensive Organic Name Reactions and Reagents. Volume 3, John Wiley & Sons, Hoboken 2009, ISBN 978-0-471-70450-8 , pp. 2442-2445.
- ^ Obituary Adrian Brook. In: Toronto Star. accessed on July 27, 2017.
- ↑ GM Brook, DM Macrae: 1,4-Silyl rearrangements of siloxyalkenes to siloxyketones during peroxidation. In: Journal of Organometallic Chemistry . Volume 77, No. 2, 1974, pp. C19-C21, doi: 10.1016 / S0022-328X (00) 81332-7 .
- ^ Jacques Cattell Press (Ed.): American Men & Women of Science. Volume 3: G-I. 16th edition. RR Bowker Company , New York 1986, ISBN 0-8352-2224-1 , p. 555.
- ^ A. Hassner, RH Reuss, HW Pinnick: Synthetic methods. VIII. Hydroxylation of carbonyl compounds via silyl enol ethers. In: The Journal of Organic Chemistry . Volume 40, No. 23, 1975, pp. 3427-3429, doi: 10.1021 / jo00911a027 .
- ^ Jacques Cattell Press (Ed.): American Men & Women of Science. Volume 6: Q-S. 16th edition. RR Bowker Company, New York 1986, ISBN 0-8352-2228-4 , p. 345.
- ↑ GM Rubottom, MA Vazquez, DR Pelegrina: Peracid oxidation of trimethylsilyl enol ethers: A facile α-hydroxylation procedure. In: Tetrahedron Letters . Volume 15, No. 49-50, 1974, pp. 4319-4322, doi: 10.1016 / S0040-4039 (01) 92153-7 .
- ↑ a b J. Clayden, N. Greeves, S. Warren: Organic Chemistry. 2nd Edition. Oxford University Press, New York 2012, ISBN 978-0-19-927029-3 , p. 466.
- ↑ J. Clayden, N. Greeves, S. Warren: Organic Chemistry. 2nd Edition. Oxford University Press, New York 2012, ISBN 978-0-19-927029-3 , p. 550.
- ↑ J. Christoffers, A. Baro, T. Werner: α-Hydroxylation of β-Dicarbonyl Compounds. In: Advanced Synthesis & Catalysis . Volume 346, No. 2-3, 2004, pp. 143-151, doi: 10.1002 / adsc.200303140 .
- ↑ M. Mandal, H. Yun, GB Dudley, S. Lin, DS Tan, SJ Danishefsky: Total Synthesis of Guanacastepene A: A Route to Enantiomeric Control. In: The Journal of Organic Chemistry . Volume 70, No. 26, 2005, pp. 10619-10637, doi: 10.1021 / jo051470k .
- ↑ CF Thompson, TF Jamison, EN Jacobsen: Total Synthesis of FR901464. Convergent Assembly of Chiral Components Prepared by Asymmetric Catalysis. In: Journal of the American Chemical Society . Volume 122, No. 42, 2000, pp. 10482-10483, doi: 10.1021 / ja0055357 .