Great test
The Tollens sample (named after the agricultural chemist Bernhard Tollens ) or the silver mirror sample is evidence of aldehydes or reducing functional groups .
The Tollens reagent is an ammoniacal silver nitrate solution , which is produced by adding concentrated ammonia solution dropwise to a silver nitrate solution until the brown precipitate of silver (I) oxide (Ag 2 O) in the soluble diammine silver ( I) complex ([Ag (NH 3 ) 2 ] + ) passes over.
For detection, add the ammoniacal silver nitrate solution to an aqueous solution of the substance to be tested in the test tube and heat it in a water bath to approx. 70 ° C for a few minutes.
The proof is positive if the solution turns black due to the precipitation of elemental silver and silver is deposited on the inner wall of the test tube, which leads to the formation of a reflective coating.
The reaction equation for the oxidation of ethanal (acetaldehyde) as an example of a reducing substance with ammoniacal silver nitrate solution to acetic acid is:
The Tollensprobe can also easily be used to detect sugars that reduce aldehyde groups , such as glucose or lactose . Although the open-chain form (aldehyde) of these sugars in aqueous solution is only present in a very small proportion in addition to the closed ring forms (here the aldehyde group is bound as a hemiacetal), the reaction is still practically complete, since the open-chain form consists of a chemical equilibrium from the Ring shapes is modeled.
z. B .:
The ammoniacal silver nitrate solution must always be freshly prepared, because the highly explosive silver nitride Ag 3 N would deposit if it was left standing for a long time .
The great test - a redox reaction
Since the sample substance is oxidized by reducing the silver (I) ions, the overall reaction, as with all redox reactions , can be broken down into an oxidation and reduction reaction . For the sake of simplicity, the following example does not take into account that the silver ions are actually present in a silver diammine complex:
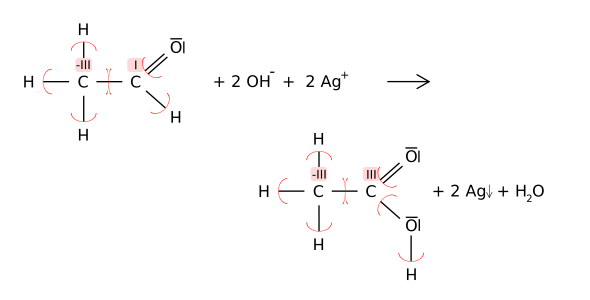
The redox reaction can also be (here with the carbon - atom ) using the oxidation numbers follow:
Since the reaction takes place in an alkaline medium, the resulting carboxy group (in the example the carboxy group of acetic acid ) is deprotonated by hydroxide ions to form the carboxylate group in the sense of an acid-base reaction . In the example, the acetate is created :
Further detection reactions for aldehydes :
- Fehling's trial
- Schiff's sample with the Schiff's reagent
- Benedict reagent
Individual evidence
- ↑ B. Tollens: About ammoniacal silver solution as a reagent for aldehyde . In: Reports of the German Chemical Society . 15, 1882, pp. 1635-1639.
- ↑ Differentiation between aldehydes and ketones
- ↑ Prof. Blume's tip of the month December 2000

![\ mathrm {CH_3CHO + 2 \ [Ag (NH_3) _2] ^ + + 2 \ OH ^ - \ longrightarrow}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5138651644387d20108d16056a8d6d5568e801df)