Spherulite

The term spherolite (from ancient Greek σφαῖρα sphaira 'ball' and ancient Greek λίθος líthos 'stone') generally refers to a spherical or radiant crystal aggregate. In Polymer Physics one for spherulite is used as thermoplastic plastics typical spherical superstructure unit referred to, are arranged radially symmetrically in the crystallites and firmly connected by amorphous intermediate areas. The size and the number of spherulites in a workpiece have a significant influence on its mechanical and optical properties.
Emergence
Spherulites can form when melts of thermoplastics cool down. The tendency to crystallize is of the type of polymer; H. depends on the arrangement of the atoms or the functional groups in the molecular chain. Examples of polymers that can form spherulite structures are: polyamide 6 , polypropylene (PP) , polyoxymethylene (POM) , polyethylene (PE) or polybutylene terephthalate (PBT) . In these polymers, molecular chains can arrange themselves regularly with one another and thus form crystallites.
The crystalline structures arise primarily from crystallization nuclei and grow uniformly outwards from their center in all directions. Therefore, spherical, radially symmetrical arrangements are formed. Whether spherulites are formed and how big they become depends on the type of polymer and the cooling conditions in the melt.
If the cooling rate is slow, fewer spherulites are formed. At the same time, however, these have a lot of time to grow and are therefore relatively large. With rapid cooling, crystallization starts in many places at the same time. Since the crystallization temperature is fallen below faster with rapid cooling, the spherulites remain comparatively small. In the case of some polymers such as polyamide (PA) or polybutylene terephthalate (PBT), relatively rapid cooling on the surface can lead to a layer with less crystallinity or even amorphous structures in the edge area.
Spherulites can only grow as long as they are surrounded by amorphous material. If the spherulites become so large that they touch, they cannot expand further in this direction. Flat surfaces are then created between the spherulites.
Foreign substances and impurities have a decisive influence on spherulite formation. They can act as nucleating agents and thus ensure increased spherulite formation. In practice, therefore, nucleating agents are sometimes added to the polymer in order to significantly accelerate the crystallization. At the same time, the solidification takes place at a higher temperature, which has an advantageous effect on process times during injection molding . Typical concentrations of nucleating agents are 0.1-0.5%. Insoluble inorganic fillers such as metal oxides, metal salts, silicates or boron nitride with particle sizes of approx. 3 μm are often added to the polymer as nucleating agents. Fillers, reinforcing agents and colorants can also have a nucleating effect. There are also nucleating agents known as “clarifiers” that are dissolved in the melt. The nucleation density here is orders of magnitude higher than with undissolved additives, so that optically much more transparent materials are created. Nucleating agents are mostly found empirically and are optimized for a specific polymer, i.e. H. a nucleating agent for, for example, polypropylene will not necessarily work for polyethylene.
Structure and structure
Spherolites are not themselves crystals in the crystallographic sense, but represent aggregates (accumulations) of very many, smaller crystalline areas. This could be demonstrated in individual materials by X-ray diffraction . The size of the spherulites alone says nothing about the crystallinity of the material (proportion crystalline to amorphous) nor about the size of the actual crystals. Rather, the spherulite size is an indication of the crystallization conditions in the polymer.
The crystallites are arranged radially symmetrically around the center. X-ray diffraction experiments of the smallest areas have shown that the polymer chains in the spherulites are arranged more or less tangentially . The mechanism of growth in which chains are gradually added one after the other would correspond to the mechanism of crystallization of short-chain paraffins. The parallel arrangement of the chains leads to birefringent properties (form birefringence) in the crystals . H. the refractive index in the radial direction differs from the tangential direction.
In addition to spherulitic superstructures, dendritic superstructures are also known for some polymers (for example polypropylene). They form when there is a strong temperature gradient in the sample. The crystallization begins in the colder area and the crystalline areas grow towards the areas with higher temperature. This leads to directed, dendritic crystal growth.
Effects
Spherulites influence the thermal properties of the polymer ( e.g. melting point , heat resistance , shrinkage), the mechanical strength and, in some cases, the chemical resistance and the optical properties.
The crystalline parts are harder, more brittle and have a higher density, while the amorphous parts are more ductile and less dense and take on the task of elasticity in the component. They also have a higher melting point, which leads to better heat resistance of the component.
The spherulites differ in their optical properties from the amorphous areas. Since they are only in rare cases significantly smaller than the wavelength of visible light, partially crystalline polymeric materials usually appear milky to opaque (opaque).
Depending on the point in time at which the spherulites collide, very firm or even no connections are created, so that the interfaces between the spherulites can form pronounced structural weak points. Large spherulites are therefore not desirable. They are avoided through nucleation and / or undercooling, so that many spherulites grow at the same time.
A reduction in the spherulite size due to increased nucleation leads to a greater flexural modulus and an increased yield point as well as lower elongation at break and ductility. A nucleation (higher proportion of nucleating agents) increases the heat resistance.
proof
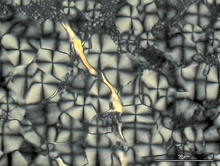
Since spherulites contain crystalline areas and are therefore birefringent, they can be detected with the help of polarization microscopy. The appearance is different and depends on the polymer used. They can usually be recognized by the typical pattern (" Maltese cross "), the dark bars of which are aligned parallel to the polarization direction of the polarizer and analyzer of the microscope. If the object is rotated, the orientation of the contrast remains in the same spatial direction, i.e. it does not rotate with the sample.
The spherulite diameter denotes the largest diameter of the 3-dimensional spherulites. The size that can be detected by light microscopy is between 1 µm and several 100 µm. With very small spherulites, the pattern described above can no longer be seen in the microscope. You can only see a diffuse scattering of the light.
literature
- Gottfried W. Ehrenstein: Polymer materials: structure properties application , Hanser Verlag, ISBN 3446211616 .
- DA Hemsley: Applied polymer light microscopy , Elsevier Applied Science, London, 111-149, ISBN 1-85166-335-5 .
Web links
- Video on the crystallization of polypropylene (spherulite formation under a polarizing microscope) on YouTube
Individual evidence
- ↑ a b Linda C. Sawyer and David T. Grubb: Polymer Microscopy , Chapman and Hall, London, 200-202, ISBN 0-412-25710-6 .
- ↑ a b c d e f D.A. Hemsley: Applied polymer light microscopy , Elsevier Applied Science, London, 111-149, ISBN 1-85166-335-5 .
- ↑ a b Nucleating agents , Nemitz plastic additives (pdf; 43 kB).
- ↑ a b Tim A. Osswald, Ernst Schmachtenberg, Sigrid Brinkmann, Erwin Baur: Saechtling Kunststoff Taschenbuch , Hanser Fachbuch, ISBN 3-446-22670-2 (reading sample pdf; 209 kB).
- ↑ Nucleating agents , Kunststofftechnik Ulrike Lapacz.
- ↑ a b Videos and explanations on the dendritic crystallization of polypropylene .
- ↑ Practical course in materials technology, microscopy of material structures / structures, Institute for Materials Technology - University of Kassel (pdf; 2.1 MB).
- ↑ Georg Menges, Edmund Haberstroh, Walter Michaeli , Ernst Schmachtenberg (2002): Material Science Kunststoffe Hanser Verlag, ISBN 3-446-21257-4 , ( Google Books ).
- ↑ W. Lutz: Improved heat resistance of thermoplastic multiphase materials through controlled crystal growth . Institute for Plastics Science and Technology, University of Stuttgart, 2005 (pdf; 1.1 MB).
- ↑ Inga Gurke: Smectic Thermotropic Main Chain Polyesterimides . Dissertation, Institute for Macromolecular and Technical Chemistry, University of Hamburg, 1999 (pdf).