Steviol
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
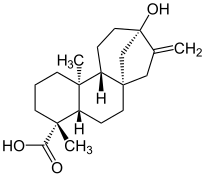
|
|||||||||||||
| General | |||||||||||||
| Surname | Steviol | ||||||||||||
| other names |
(-) - 13-Hydroxykaur-16-en-18-acid |
||||||||||||
| Molecular formula | C 20 H 30 O 3 | ||||||||||||
| Brief description |
crystalline solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 318.45 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
215 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Steviol is a naturally occurring, chiral diterpene from the group of Kauranes or Kaurene . It is found in the leaves of the South American plant Stevia rebaudiana in the form of various glycosides ( stevioside ). The glycosides have a very sweet taste. In contrast to the strongly sweet stevioside, steviol is tasteless.
Occurrence
Steviol is found in stevia in the form of its glycosides . Up to 60 g of glycosides can be extracted from 1 kg of the dried drug.
Extraction and presentation
Steviol can be obtained from the glycosides by enzymatic hydrolysis with the enzyme diastase . The acid-catalyzed hydrolysis fails because steviol is rearranged into isosteviol .
properties
Physical Properties
The optical rotation value [α] D is −65 ° (CHCl 3 ).
Chemical properties
Steviol is temperature resistant up to 200 ° C.
Biological importance
Steviol is structurally related to gibberellins . Accordingly, it has a weak growth-promoting effect. A mutant of the fungus Gibberella fujikuroi converts steviol into 13-hydroxygibberellin. Steviol inhibits oxidative phosphorylation in rat mitochondria and acts as a repellent against the aphid Schizapis graminum .
toxicity
Ames test (S9 activation, chromosome aberration test, micronucleus test and HPRT test): Steviol was found to be weakly mutagenic and genotoxic in in vitro studies. The effect is attributed to metabolites of steviol - such as 15-Oxosteviol. Due to a lack of data, steviol glycosides have not yet been approved as sweeteners in North America.
proof
Steviol can be analyzed by HPLC.
Individual evidence
- ↑ a b c d Entry on steviol. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ John R. Bearder, Valerie M. Frydman, Paul Gaskin, Jake MacMillan, Colin M. Wels, Bernard O. Phinney: Fungal products. Part XVI. Conversion of isosteviol and steviol acetate into gibberellin analogues by mutant B1–41a of Gibberella fujikuroi and the preparation of [ 3 H] gibberellin A 20 . In: J. Chem. Soc., Perkin Trans. 1 1976, pp. 173-174. doi : 10.1039 / P19760000173 .
- ↑ Vignais, PV et al. : Effects of atractyligenin and its structural analogues on oxidative phosphorylation and on the translocation of adenine nucleotides in mitochondria. in: Biochim Biophys Acta . 1966 Jun 15; 118 (3): pp. 465-483; PMID 4226320 .
- ↑ Nanayakkara, NP et al. : Characterization and feeding deterrent effects on the aphid, Schizaphis graminum, of some derivatives of the sweet compounds, stevioside and rebaudioside A. in: J Nat Prod . 1987 May-Jun; 50 (3): pp. 434-441; PMID 3668559 .
- ↑ Medon, PJ et al. : Safety assessment of some Stevia rebaudiana sweet principles. in Fed. Proc. 41: p. 1568, 1982.
- ↑ Suttajit, M. et al. : Mutagenicity and human chromosomal effect of stevioside, a sweetener from Stevia rebaudiana Bertoni , in: Environ Health Perspect . 1993 Oct; 101 Suppl 3: pp. 53-56; PMID 8143647 PMC 1521159 (free full text, PDF).
- ↑ Terai, T. et al. : Mutagenicity of steviol and its oxidative derivatives in Salmonella typhimurium TM677. in Chem Pharm Bull . 2002 Jul; 50 (7): pp. 1007-1010; PMID 12130868 , doi: 10.1248 / cpb.50.1007 .
- ↑ Harriet Wallin: steviol glycosides - Chemical and Technical Assessment. 63rd JECFA 2004 (PDF)
