Tetraammine copper sulfate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
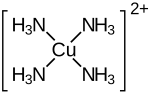  |
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tetraammine copper sulfate | ||||||||||||||||||
| other names |
Tetrammine copper (II) sulfate |
||||||||||||||||||
| Molecular formula | [Cu (NH 3 ) 4 ] SO 4 • H 2 O | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 245.75 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.81 g cm −3 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tetraammine copper (II) sulfate [Cu (NH 3 ) 4 ] SO 4 , (former names: Cuprum sulfuricum ammoniatum , sulfuric acid copper oxide ammonia, copper almia, cupric ammonium sulfate, tetrammine copper (II) sulfate) is a complex salt of divalent copper and sulfuric acid . As a solid, it is present as a monohydrate [Cu (NH 3 ) 4 ] SO 4 · H 2 O. The deep blue color of the salt crystals is caused by the tetraammine complex [Cu (NH 3 ) 4 ] 2+ of copper, which is complexed by two additional water molecules in aqueous solution. The salt, which slowly weathered in air to a green powder, has a decomposition point of approx. 150 ° C.
Tetraammine copper (II) sulfate is formed by adding an aqueous copper (II) sulfate solution with excess ammonia water:
The color change from pale blue to deep blue can be used to detect the copper ions. The use of copper salts as a secret ink is based on the formation of the tetraammine copper complex .
Although the tetraammine copper (II) salts are stable complex salts, a reaction with hydrogen sulfide (H 2 S) is possible: When adding hydrogen sulfide (H 2 S), black copper (II) sulfide is precipitated. The copper (II) ion concentration resulting from the equilibrium of the complex according to the equation
is higher than the max. Solution concentration of the copper (II) sulfide . Therefore, despite the complex compound, copper (II) sulfide is precipitated.
When milk of lime is added to a solution of tetraammine copper (II) sulfate, a blue-green precipitate forms. This color pigment, also known as lime blue or new blue , is used for painters' paints.
Tetraammine copper sulfate was first synthesized in 1644 by Johan Baptista van Helmont .
The aqueous solution was introduced as "volatile copper oxide tincture" (Tinctura veneris volatilis) by Herman Boerhaave (1668–1738), but was no longer used.
Individual evidence
- ↑ a b c Datasheet Tetraamminecopper (II) sulfate monohydrate from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ Jander-Wendt: Textbook of analytical and preparative inorganic chemistry , 3rd edition, S. Hirzel Verlag, Sttg., 1959, p. 243.
- ↑ Brockhaus der Naturwissenschaften und der Technik , 4th edition, SFABrockhaus Verlag, Wiesbaden, 1958, p. 325.

![{\ displaystyle \ mathrm {CuSO_ {4} +4 \ NH_ {3} + H_ {2} O \ longrightarrow \ {[Cu (NH_ {3})} _ {4}] SO_ {4} \ cdot \ H_ { 2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d487fa42ca6b123db865fbc2b9acd8fe3dcbbc47)

![{\ displaystyle \ mathrm {[Cu (NH_ {3}) _ {4}] ^ {\, 2 +} \ rightleftharpoons \ Cu ^ {\, 2 +} + 4 \ NH_ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/49ec40818e0d3ae14ce8fd072495a0fd29090ea2)