Triglycine sulfate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triglycine sulfate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 17 N 3 O 10 S | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 323.28 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.69 g cm −3 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
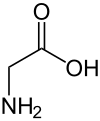
Triglycine sulfate ( TGS ) can formally be understood as an adduct of three moles of glycine and one mole of sulfuric acid . TGS single crystals are used as detector material in pyroelectric sensors .
application
Triglycine sulfate has a large pyroelectric coefficient . If there is a temperature change, for example due to the absorption of electromagnetic waves, the polarization changes and a charge is generated which can be measured immediately after amplification. For this purpose, the respective single crystal surfaces must be provided with suitable electrodes. Temperatures can also be measured with a similar arrangement. The Curie temperature of TGS is 49 ° C. Below this temperature, the polarization changes when the incident radiation power changes, which can be measured in the form of a voltage surge.
Deuterated triglycine sulfate
Deuterated triglycine sulfate ( DTGS ) differs from TGS in that all hydrogen atoms are completely substituted by deuterium atoms. The empirical formula is C 6 D 17 N 3 SO 10 . DTGS has the CAS no. 17237-73-9 and, as a crystalline solid, has a molar mass of 340.18 g mol −1 .
DTGS also has pyroelectric properties. Compared to TGS, it has the advantage of a higher Curie temperature, depending on the degree of deuteration, this is 57–62 ° C, instead of 49 ° C for TGS. The difference in Curie temperature has a major impact on the areas of application of DTGS-based sensors. For example, direct sunlight can heat a sensor to 50 ° C. Above the Curie temperature, however, the pyroelectric properties are lost and the detector loses its function. DTGS sensors therefore offer a wider range of applications than TGS detectors, especially in the case of passively cooled operation.
A further improvement in the pyroelectric properties can be achieved by doping the DTGS crystals with L -alanine , so-called deuterated L -alanine-doped triglycine sulfate (DLaTGS). The alanine doping can increase the sensitivity of the DTGS crystals on the one hand and reduce the permanent depolarization when heated above the Curie temperature on the other.
Due to their material properties, DTGS and DLaTGS have gained importance as sensor materials in infrared spectroscopy .
Individual evidence
- ↑ Triglycine sulfate data sheet from Acros, accessed on February 20, 2010.
- ↑ EA Wood, AN Holden: Monoclinic glycine sulfate: crystallographic data. In: Acta Cryst. 1957, 10, pp. 145-146, doi: 10.1107 / S0365110X57000481 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Patent EP0011808 : Pyroelectric component for measuring the intensity of electromagnetic radiation, for temperature measurement and for thermoelectric power generation and use of this component. Inventor: Siegfried Haussühl, Josef Liebertz (description of TGS in points 0002 and 0003).
- ↑ a b Triglycine sulfate (TGS) & Deuterated triglycine sulfate (DTGS) ( Memento of November 24, 2009 in the Internet Archive ). GIRMET Ltd.
- ↑ DTGS detectors in the lexicon of IR spectroscopy