Uranium (V) bromide
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| __ U 5+ __ Br - | ||||||||||
| General | ||||||||||
| Surname | Uranium (V) bromide | |||||||||
| other names |
Uranium pentabromide |
|||||||||
| Ratio formula | UBr 5 | |||||||||
| Brief description |
deep brown solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 637.55 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| solubility |
sparingly soluble in bromine |
|||||||||
| Hazard and safety information | ||||||||||
 Radioactive |
||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Uranium (V) bromide is an inorganic chemical compound from the group of bromides .
presentation
Uranium (V) bromide can be obtained by the catalytic reaction of uranium with bromine under acetonitrile or by the reaction of uranium (IV) bromide with bromine.
properties
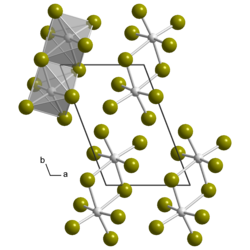
Uranium (V) bromide is a deep brown, hygroscopic , crystalline solid. Its crystal structure is isotypic with the triclinic of β- uranium (V) chloride with the space group P 1 (space group no. 2) . It decomposes on contact with water.
Individual evidence
- ↑ a b c d Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 1214.
- ↑ Entry on uranium compounds in the GESTIS substance database of the IFA , accessed on February 1, 2016 (JavaScript required)
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry uranium compounds with the exception of those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) , accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling.
- ^ Norman M. Edelstein, Lester R. Morss, Jean Fuger: The Chemistry of the Actinide and Transactinide Elements . Springer, 2010, ISBN 94-007-0211-6 , pp. 526, 1795 ( limited preview in Google Book search).



