Prohibited transition

In physics, a forbidden transition is a transition from one energy level - or more generally from one (quantum mechanical) state - to another if it does not occur at all or if it occurs much less frequently than other transitions in the same system.
"Prohibition" by physical law
In the narrower sense, forbidden transitions are those that cannot occur according to a natural law . The existence of such transitions would be a violation of natural law and thus an indication of its limits of validity. Which transitions are allowed and which are forbidden is sometimes formulated in selection rules .
Accordingly, there are forbidden decays . For example, a carbon -12 atomic nucleus would have to be supplied with energy from the outside in order to convert it into one of the nuclei nitrogen -12 or boron -12, because their mass is greater (see mass-energy equivalence ). The spontaneous transition - the beta decay - is "energetically forbidden" here, ie it would contradict the law of conservation of energy .
"Forbidden" in the sense of "rarely observed"
Transitions that occur only very rarely under laboratory conditions , although they do not contradict any physical law, are also referred to as prohibited . The reason for the rarity can lie , for example, in the “competition” of another target state with a higher transition probability or in the competition with another mechanism for changing the state (see following section).
Prohibited emission line
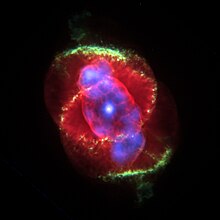

For some astronomical objects, such as the Cat's Eye Nebula , green emission lines have been observed, which arise from the radiating de-excitation of a metastable energy level . Since they cannot usually be produced in the laboratory on earth, it was initially assumed that an element called nebulium, which was previously unknown on earth, was the source of these lines. One example in the earth's atmosphere are the “forbidden” green oxygen lines of the northern lights .
The origin of such forbidden lines was explained by Ira S. Bowen in 1927 with Nebulium: Under terrestrial conditions, the atoms or molecules are de-excited by collisions before they can emit their excitation energy . Below a particle density of 10 8 / cm 3 , however, the mean time between the collisions of the particles with one another is greater than the occupation time of the metastable state, and the transition with radiation emission becomes probable. Hence, the forbidden lines occur in highly dilute gases such as those of the upper atmosphere or in the interstellar medium .
When describing a spectrum, forbidden lines are indicated by square brackets around the emitting atom or ion .
Some of the most prominent emission lines from H-II areas and planetary nebulae are such forbidden lines, such as the [O III] line of doubly ionized oxygen at 500.7 nm or the [N II] line of singly ionized nitrogen at 658 .3 nm. The solar corona shows particularly strong forbidden lines of highly charged ions , e.g. B. the "green" line of the 13-fold positively charged Fe 13+ ion, [Fe XIV] at 530.3 nm.
Individual evidence
- ↑ ?. P. Raju et al: The Excitation Mechanism of [Fe XIV] 5303 Å Line in the Inner Regions of Solar Corona . In: J. Astrophys. Astr. (1991) 12, 311-317