Tungsten (II) iodide
| Crystal structure | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| __ W 2+ __ I - | |||||||||||||
| General | |||||||||||||
| Surname | Tungsten (II) iodide | ||||||||||||
| other names |
Tungsten diiodide |
||||||||||||
| Ratio formula | WI 2 | ||||||||||||
| Brief description |
ocher to black solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 437.65 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| density |
6.79 g cm −3 |
||||||||||||
| Melting point |
800 ° C (decomposition) |
||||||||||||
| solubility |
almost insoluble in water |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Tungsten (II) iodide is an inorganic chemical compound of tungsten from the group of iodides .
Extraction and presentation
Tungsten (II) iodide can be obtained by decomposing tungsten (III) iodide .
It is also possible to obtain it from tungsten (II) chloride by halogen exchange reaction
or by reaction of tungsten (VI) chloride with hydrogen iodide at 110 ° C and subsequent degradation at 500 ° C in vacuo.
It is also formed reversibly when iodine reacts with tungsten, which is used to extend the service life of halogen lamps .
The reaction of tungsten hexacarbonyl with iodine also produces tungsten (II) iodide.
properties
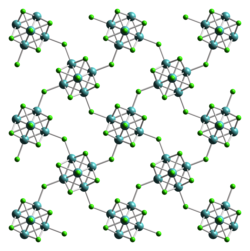
Tungsten (II) iodide is an ocher-colored to black solid that is stable in air and to moisture at room temperature. It has a crystal structure isotypic to that of tungsten (II) chloride, it crystallizes orthorhombically in the space group Bbem (space group no.64 , position 5) with the lattice parameters a = 1258 pm, b = 1259 pm, c = 1584 pm.
Individual evidence
- ↑ a b c d e f Georg Brauer: Handbook of preparative inorganic chemistry . 3., reworked. Edition. tape III . Enke, Stuttgart 1981, ISBN 3-432-87823-0 , pp. 1564 .
- ↑ a b c W. M. Haynes (Ed.): CRC handbook of chemistry and physics. A ready-reference book of chemical and physical data . founded by David R. Lide. 93rd edition. CRC Press, Boca Raton 2012, ISBN 978-1-4398-8049-4 , pp. 4–96 (English, limited preview in Google Book Search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Hans Peter Latscha, Martin Mutz: Chemistry of the elements . Springer, 2011, ISBN 3-642-16915-5 , pp. 231 ( limited preview in Google Book search).
- ^ BFG Johnson: Inorganic Chemistry of the Transition Elements . Royal Society of Chemistry, 1972, ISBN 0-85186-500-3 , pp. 94 ( limited preview in Google Book search).
- ↑ The former name of this group of rooms was Bbam .
![{\ displaystyle \ mathrm {6 \ WI_ {3} \ longrightarrow [W_ {6} I_ {8}] I_ {4} +3 \ I_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ec19d523ff56f79ea3340a9205e01572e63cee26)
![{\ displaystyle \ mathrm {[W_ {6} Cl_ {8}] Cl_ {4} +12 \ I ^ {-} \ longrightarrow [W_ {6} I_ {8}] I_ {4} + 12Cl ^ {-} }}](https://wikimedia.org/api/rest_v1/media/math/render/svg/64ca82c61b3221b87a0a288d53d82919f22e7596)
