Tungstophosphoric acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
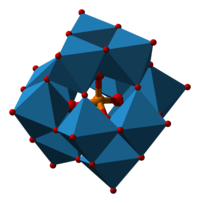
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tungstophosphoric acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | H 3 PW 12 O 40 | |||||||||||||||
| Brief description |
white or slightly yellowish green crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 2880.2 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
95 ° C |
|||||||||||||||
| solubility |
soluble in water, ethanol and diethyl ether |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tungstophosphoric acid is a phosphoric acid - tungsten compound and belongs to the heteropoly acids .
Extraction and presentation
The compound can be obtained by reacting sodium tungstate with phosphoric acid .
properties
Tungstophosphoric acid crystallizes as a hydrate with 44 molecules of water in the crystal lattice.
use
Tungstophosphoric acid is used as Scheibler's reagent for the detection of alkaloids (yellow precipitate) and as the Folin-Ciocalteu reagent for the detection of uric acid , phenol or ascorbic acid. Other areas of application include antistatic finishing in the textile industry, printing inks, paper dyes and the pigmentation of waxes. It is also used in histology to color the connective tissue.
Individual evidence
- ↑ a b c d e Entry on 12-tungstophosphoric acid. In: Römpp Online . Georg Thieme Verlag, accessed on January 20, 2014.
- ↑ a b c Datasheet Phosphotungstic acid hydrate from Sigma-Aldrich , accessed on January 20, 2014 ( PDF ).
