Xylenols
In chemistry, the xylenols or dimethylphenols form a group of aromatic compounds with two methyl groups and one hydroxyl group . They can be regarded as hydroxy derivatives of xylenes or dimethyl derivatives of phenol. There are therefore six isomers . The name xylenol is derived from the combination of the names of the aromatic compounds xylene and phenol .
Structure and properties
The physical properties of the six xylenol isomers are similar. The xylenols are usually only very sparingly soluble in water. They form colorless to yellowish needles or plates with a pungent odor. Chemically, they behave like typical phenols, for example in alkaline solutions they form phenolates, which are significantly more soluble in water.
The methyl groups have a (weak) + I effect on the aromatic, which increases the electron density in the ring. This u. a. the acidity of the phenolic OH is weakened. The pK s values will be somewhat higher than that of phenol (9.99) and the cresols .
| Xylenols | |||||||||||
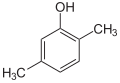
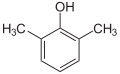
| Surname | 2,3-xylenol | 2,4-xylenol | 2,5-xylenol | 2,6-xylenol | 3,4-xylenol | 3,5-xylenol | |||||
| other names | 2,3-dimethylphenol , vic.- o -xylenol |
2,4-dimethyl phenol, asym.- m -Xylenol |
2,5-dimethylphenol , p -xylenol |
2,6-dimethylphenol , vic.- m -xylenol |
3,4-dimethylphenol , asym.- o -xylenol |
3,5-dimethylphenol , sym.- m -xylenol |
|||||
| Structural formula |

|

|
|
|

|

|
|||||
| CAS number | 526-75-0 | 105-67-9 | 95-87-4 | 576-26-1 | 95-65-8 | 108-68-9 | |||||
| 1300-71-6 (mixture of isomers) | |||||||||||
| PubChem | 10687 | 7771 | 7267 | 11335 | 7249 | 7948 | |||||
| Molecular formula | C 8 H 10 O | ||||||||||
| Molar mass | 122.17 g mol −1 | ||||||||||
| Physical state | solid, isomer mixture mostly liquid | ||||||||||
| Brief description | colorless to yellowish needles or plates with a pungent odor | ||||||||||
| Melting point | 73-75.5 ° C | 24 ° C | 75-77 ° C | 46-48 ° C | 65-68 ° C | 61 ° C | |||||
| boiling point | 216 ° C | 210 ° C | 212 ° C | 203 ° C | 226 ° C | 219 ° C | |||||
| pK s value | 10.50 | 10.45 | 10.22 | 10.59 | 10.32 | 10.15 | |||||
| solubility | Slightly soluble in water (2,6-xylenol: readily to very readily soluble in water, 3,4-xylenol: miscible), readily soluble in ethanol and ether |
||||||||||
|
GHS labeling |
|
|
|||||||||
| H and P phrases | 301-311-314-411 | 301-311-314 | |||||||||
| no EUH phrases | no EUH phrases | ||||||||||
|
273-280 305 + 351 + 338-310 |
280-273 302 + 352-301 + 330 + 331 305 + 351 + 338-309 + 310 |
273-280 309 + 310-302 + 352 305 + 351 + 338 |
280-273 302 + 352-301 + 330 + 331 305 + 351 + 338-309 + 310 |
273-280 301 + 330 + 331-302 + 352 305 + 351 + 338-309 + 310 |
280 305 + 351 + 338-310 |
||||||
Occurrence
All xylenols are found in coal tar and beech wood tar and are part of the creosote mixture .
presentation
As a rule, xylenols can be prepared from the xylidines by diazotization and subsequent boiling of the diazonium salt.
use
Together with the cresols and cresolic acids , xylenols are an important class of phenols with great industrial importance. Xylenols are used as starting materials for the synthesis of pesticides , antioxidants and pharmaceuticals (e.g. mexiletine ). 2,5-Xylenol ( p -Xylenol) serves as the basis for the representation of the pH indicators xylenolphthalein , xylenol blue and bromoxylenol blue . Metal phthalein and xylenol orange are derived from 2,6-xylenol ; they are used as indicators in complexometry . 2,6-Xylenol is used as a starting material for the synthesis of polyphenylene ether . The disinfectant chloroxylenol is accessible by chlorinating 3,5-xylenol .
Web links
Individual evidence
- ↑ a b c Entry for CAS no. 1300-71-6 in the GESTIS substance database of the IFA , accessed on December 15, 2013(JavaScript required) .
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ a b c d Entry for CAS no. 526-75-0 in the GESTIS substance database of the IFA , accessed on December 15, 2013(JavaScript required) .
- ↑ a b c d Entry for CAS no. 105-67-9 in the GESTIS substance database of the IFA , accessed on December 15, 2013(JavaScript required) .
- ↑ a b c d Entry for CAS no. 95-87-4 in the GESTIS substance database of the IFA , accessed on December 15, 2013(JavaScript required) .
- ↑ a b c d e Entry for CAS no. 576-26-1 in the GESTIS substance database of the IFA , accessed on December 15, 2013(JavaScript required) .
- ↑ a b c d e Entry for CAS no. 95-65-8 in the GESTIS substance database of the IFA , accessed on December 15, 2013(JavaScript required) .
- ↑ a b c d Entry for CAS no. 108-68-9 in the GESTIS substance database of the IFA , accessed on December 15, 2013(JavaScript required) .
- ↑ HPT Ammon, C. Hunnius: Hunnius pharmaceutical dictionary , Verlag Walter de Gruyter 2004, ISBN 3-11-017475-8 , p. 867.
- ↑ Hager's Handbook of Pharmaceutical Practice , p. 921 ( limited preview in Google book search).


