Polyphenylene ether
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Polyphenylene ether | ||||||
| other names |
|
||||||
| CAS number | 25134-01-4 | ||||||
| Monomer | 2,6-xylenol | ||||||
| Molecular formula of the repeating unit | C 8 H 8 O | ||||||
| Molar mass of the repeating unit | 120.15 g mol −1 | ||||||
| Type of polymer | |||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| density |
1.06 g cm −3 |
||||||
| Glass temperature |
215 ° C |
||||||
| modulus of elasticity |
2000 MPa (Vestoran 1090) |
||||||
| Chemical resistance |
diluted acids, strong alkalis, alcohol, detergents |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
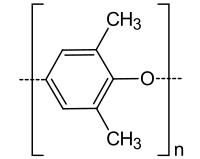
Polyphenylene ether ( abbreviation PPE , also poly (oxy-2,6-dimethyl-1,4-phenylene) or polyether, formerly called polyphenylene oxide, PPO ) is a high-temperature-resistant, thermoplastic material with the general formula (C 8 H 8 O) n . Technically, it is produced by the oxidative coupling of 2,6-dimethylphenol . In its pure form, PPE is rarely used; it is mainly used as a blend with polystyrene , impact-resistant styrene-butadiene copolymer or polyamide .
history
Polyphenylene ether was discovered by Allan S. Hay in 1956 and introduced to the market by General Electric since 1960 . The common name polyphenylene oxide (PPO) is wrong, as it is not an oxide, but an ether.
Although it was considered to be one of the most cost-effective, high-temperature-resistant plastics, processing was difficult and the impact strength and temperature resistance decreased over time. By mixing with polystyrene in any ratio, the disadvantages could be compensated and the properties could be modified in a targeted manner. At the end of the 1960s, modified PPE products came onto the market under the trade name Noryl.
properties
PPE is an amorphous high-performance plastic . The glass transition temperature is 215 ° C, but can be modified within a wide range by mixing with polystyrene . The properties can be modified over a wide range by modifying and adding fillers such as glass fibers .
Manufacturing
If 2,6-xylenol is reacted with copper (I) or copper (II) chloride in pyridine in the presence of atmospheric oxygen, the polyphenyl ether is formed. In the case of copper (I) chloride, it is first oxidized to copper (II) chloride by the oxygen in the air. Then there is a one-electron transfer from 2,6-xylenol to the copper (II) chloride, which is reduced to copper (I) chloride. The atmospheric oxygen oxidizes the reduced species again to form copper (II) chloride. Two 2,6-xylenol radicals can now dimerize . The resulting dimer can form a radical by one-electron transfer and react with another 2,6-xylenol radical; this reaction can be repeated n times and is called oxidative coupling . Since this reaction creates water as a condensation product, it is a polycondensation reaction .
use
PPE blends are used for molded parts in the electronics, household and vehicle sectors where high heat resistance, dimensional stability and dimensional stability are important. But they are also used in medical technology - for example for plastic instruments that are often sterilized. The PPE blends are characterized by hot water resistance with low water absorption, high impact strength, halogen-free fire protection and low density. Processing is carried out by injection molding or extrusion at a processing temperature between 260 ° C and 300 ° C, depending on the type. The surface can be printed, hot stamped, painted or metallized. Welding is possible by means of heating element, friction or ultrasonic welding. It can be glued with halogenated solvents or various adhesives.
Trade names
Trade names of polyphenylene ethers include:
- Artley (PPE + PE) from Sumitomo Chemical
- Gecet (PPE + EPS) from Huntsman
- Iupiace (PPE + PE) from Mitsubishi Engineering-Plastics
- Lemalloy (PPE + PA) from Mitsubishi Engineering-Plastics
- Luranyl (PPE + PS) from ROMIRA
- Noryl (PPE + PS) from SABIC
- Noryl EF (PPE + EPS) from SABIC
- Noryl GTX (PPE + PA) from SABIC
- Suncolor PPE (PPE + EPS) from Sunpor
- Ultranyl (PPE + PA) from BASF
- Vestoran (PPE + PS) from Evonik Degussa , formerly Chemische Werke Hüls
- Xyron (PPE + PS), (PPE + PA), (PPE + PP), (PPE + PPS), (PPE powder) from Asahi Kasei
Norms
- ASTM D 4349-1996 Polyphenylene Ether (PPE) materials
- DIN EN ISO 28941-1 Plastics - Polyphenylene ether (PPE) molding compounds - Part 1: Designation system and basis for specifications (2008)
Individual evidence
- ↑ Aldrich Chemistry Handbook Fine Chemicals. 2009/2010, p. 2212.
- ^ E. Kaisersberger, S. Knappe, H. Möhler: TA for Polymer Engineering - DSC TG DMA. In: NETZSCH Annual for Science and Industry. Vol. 2, 1993, p. 83 (Note: Other values are sometimes found in the literature, since PPE is rarely used in its pure form, but the glass transition temperature is of less importance).
- ↑ CAMPUS database, manufacturer information, as of December 16, 2002.
- ↑ Christian Krebs, Marc-André Avondet, Kurt W. Leu: Long-term behavior of thermoplastics. Carl Hanser Verlag 1999.
- ↑ Data sheet Poly (2,6-dimethyl-1,4-phenylene oxide) from Sigma-Aldrich , accessed on July 30, 2017 ( PDF ).
- ↑ D. Alberti: Modified aromatic polyethers. In: plastics. 10, 1987, p. 1001.
- ↑ Martin Bartmann, Udo Kowalczik: On the mechanism of the oxidative coupling of phenols . In: The Macromolecular Chemistry . tape 189 , no. 10 , 1988, pp. 2285–2292 , doi : 10.1002 / macp.1988.021891008 .
- ^ A. Hohmann, W. Hielscher: Lexikon der Zahntechnik: The basic work: 12,000 terms from dental technology and dentistry in one volume. Verlag Neuer Merkur, 1998, ISBN 3-929360-28-4 .
- ↑ Modified polyphenylene ethers (PPE). In: plastics. 10, 1989, p. 921.
- ^ H. Feldmann, P. Steiert: Modified polyphenylene ethers (PPE blends). In: plastics. 10, 1990, p. 1123.
- ↑ Jos van Gisbergen, Wim Minderhout: Modified polyphenylene ethers (PPE). In: plastics. 10, 2001, p. 304.
- ↑ BASF sells the business with the plastic Luranyl

