Kilogram: Difference between revisions
→The nature of mass: link revised to avoid redirect |
Ehrenkater (talk | contribs) Undid revision 1227411983 by 2409:40E4:4E:5BB4:8000:0:0:0 (talk) |
||
| Line 1: | Line 1: | ||
{{Short description|Metric unit of mass}} |
|||
{{lowercase}} |
|||
{{Redirect| |
{{Redirect|kg||KG (disambiguation){{!}}KG}} |
||
{{Use mdy dates|date=September 2022}} |
|||
[[Image:CGKilogram.jpg|thumb|right|307px|Shown above is a computer-generated image of the ''International Prototype Kilogram'' (“IPK”). The IPK ''is'' the kilogram. It sits next to an inch-based ruler for scale. The IPK is made of a [[platinum]]-[[iridium]] alloy and is stored in a vault at the [[Bureau International des Poids et Mesures|BIPM]] in [[Sèvres]], France. Like the other prototypes, the edges of the IPK have a four-angle chamfer to minimize wear (although only three can be seen in this image—even at high magnification). For other kilogram-related images, see ''[[#External links|External links]]'', below.]]<!--NOTE TO EDITORS: Before changing the picture size, editors are asked to see [[Talk:kilogram#Image Size]] on the discussion page.--> |
|||
{{Infobox unit |
|||
The '''kilogram''' or '''kilogramme''' (symbol: kg) is the [[SI base unit|base unit]] of [[mass]] in the [[International System of Units]] (known also by its French-language initials “SI”). The kilogram is defined as being equal to the mass of the ''International Prototype Kilogram''<span style="margin-left:0.15em"><ref name="FirstCGPM"> |
|||
| name = kilogram |
|||
{{cite web |
|||
| image = Poids fonte 5 kg à 2 hg 02.jpg |
|||
|publisher=BIMP |
|||
| caption = A series of 5, 2, 1, 0.5 and 0.2 kilogram weights, made out of rusty [[cast iron]] |
|||
|url=http://www.bipm.org/en/CGPM/db/1/1/ |
|||
| standard = [[SI]] |
|||
|title=Resolution of the 1st CGPM (1889) |
|||
| quantity = [[mass]] |
|||
}}</ref></span> (IPK; known also by its French-language name ''Le Grand K''<span style="margin-left:0.15em"><ref> |
|||
| symbol = kg |
|||
{{cite book |
|||
| units1 = [[Avoirdupois]] |
|||
| author = A. Rupert Hall and Marie Boas Hall |
|||
| inunits1 = ≈ {{val|2.204623}} [[pound (mass)|pounds]]<ref group="Note">The avoirdupois pound is part of both [[United States customary units|United States customary system of units]] and the [[Imperial units|Imperial system of units]]. It is [[International yard and pound|defined as exactly]] {{val|0.45359237|u=kilograms}}.</ref> |
|||
| title = A Brief History of Science |
|||
| units2 = British Gravitational |
|||
| publisher = New American Library of Canada |
|||
| inunits2 = ≈ {{val|0.0685}} [[slug (unit)|slugs]] |
|||
| year = 1964 |
|||
| units3 = [[CGS unit]]s |
|||
| pages = 6 |
|||
| inunits3 = 1000 [[gram]]s |
|||
}}</ref>),</span> which is almost exactly equal to the mass of one [[litre|liter]] of water. It is the only SI base unit with an [[SI prefix]] as part of its name. It is also the only SI unit that is still defined in relation to an [[#Glossary|artifact]] rather than to a fundamental physical property that can be reproduced in different laboratories. |
|||
| units4 = [[Atomic mass unit]]s |
|||
| inunits4 = {{val|6.02214076|e=26}} [[Dalton (unit) | Da]] |
|||
}} |
|||
The '''kilogram''' (also '''kilogramme'''<ref name=":1" />) is the [[International_System_of_Units#Base_units|base unit]] of [[mass]] in the [[International System of Units]] (SI), having the unit symbol '''kg'''. It is a widely used measure in science, engineering and commerce worldwide, and is often simply called a '''kilo''' colloquially.<ref>[https://www.merriam-webster.com/dictionary/kilo Merriam-Mebster definition of Kilo]</ref> It means 'one thousand [[Gram|grams]]'. |
|||
In everyday usage, the mass of an object in kilograms is often referred to as its [[weight]], although strictly speaking the weight of an object is the [[gravitation]]al [[force]] on it, measured in [[newton]]s (see also ''[[kilogram-force]])''. Similarly, the [[avoirdupois]] [[Pound (mass)|pound]], used in both the [[Imperial units|Imperial system]] and [[United States customary units|U.S. customary units]], is a unit of mass and its related unit of force is the [[pound-force]]. The avoirdupois pound is defined as exactly {{val|0.45359237|u=kg}},<ref>{{cite web |
|||
|publisher=NIST |
|||
|url=http://www.nist.gov/public_affairs/faqs/qmetric.htm |
|||
|title=Converting Measurements to Metric--NIST FAQs |
|||
}}<br> |
|||
{{cite web |
|||
|publisher=U.K. National Weights & Measures Laboratory |
|||
|url=http://www.nwml.gov.uk/faqs.aspx?ID=8 |
|||
|title=Metric Conversions |
|||
}}<br> |
|||
{{cite web |
|||
|publisher=NIST |
|||
|title=Fed-Std-376B, Preferred Metric Units for General Use By the Federal Government |
|||
|section=Section 5.2.1 |
|||
|section_title=Mass (weight) |
|||
|url=http://ts.nist.gov/WeightsAndMeasures/Metric/upload/fs376-b.pdf |
|||
|format=294 KB PDF |
|||
}}</ref> making one kilogram approximately equal to 2.2046 avoirdupois pounds. |
|||
The kilogram is a SI [[Base unit (measurement)| base unit]], defined in terms of two other base units, the [[second]] and the [[metre]] and the [[Planck constant]], a SI [[International_System_of_Units#SI_defining_constants| defining constant]].<ref name="SIBrochure9thEd"/>{{rp|131}} A properly equipped [[metrology]] laboratory can calibrate a mass measurement instrument such as a [[Kibble balance]] as a primary standard for the kilogram mass.<ref>{{Cite web |date=July 7, 2021 |title=Mise en pratique for the definition of the kilogram in the SI |url=https://www.bipm.org/documents/20126/41489673/SI-App2-kilogram.pdf/5881b6b5-668d-5d2b-f12a-0ef8ca437176?version=1.9&t=1637237674882&download=false |access-date=February 18, 2022 |website=BIPM.org}}</ref> |
|||
Many units in the SI system are defined relative to the kilogram so its stability is important. After the International Prototype Kilogram had been found to vary in mass over time, the [[International Committee for Weights and Measures]] (known also by its French-language initials CIPM) recommended in 2005 that the kilogram be redefined in terms of fundamental constants of nature.<ref name="94thCIPM"> |
|||
{{cite web |
|||
|title=Proceedings of the 94th meeting (October 2005) of the International Committee for Weights and Measures |
|||
|url=http://www.bipm.fr/utils/common/pdf/CIPM2005.zip |
|||
|format=1.1 MB zipfile |
|||
}}</ref> |
|||
The kilogram was originally defined in 1795 during the [[French Revolution]] as the mass of one [[litre]] of [[properties of water|water]]. The current definition of a kilogram agrees with this original definition to within 30 [[parts per million]]. In 1799, the platinum ''[[Grave (unit)#Kilogramme des Archives|Kilogramme des Archives]]'' replaced it as the standard of mass. In 1889, a cylinder of [[Platinum-iridium alloy|platinum-iridium]], the [[International Prototype of the Kilogram]] (IPK), became the standard of the unit of mass for the metric system and remained so for 130 years, before the current standard was [[2019 redefinition of the SI base units|adopted in 2019]].<ref name="vox" /> |
|||
==The nature of mass== |
|||
{{main|Mass versus weight}} |
|||
The kilogram is a unit of [[mass]], the measurement of which corresponds to the general, everyday notion of how “heavy” something is. However, mass is actually an ''[[inertia]]l'' property; that is, the tendency of an object to remain at constant velocity unless acted upon by an outside [[force]]. An object with a mass of one kilogram will [[acceleration|accelerate]] at one [[Metre per second squared|meter per second squared]] (about one-tenth the acceleration due to Earth’s gravity) when acted upon by a force of one [[newton]] (symbol: N). |
|||
== Definition == |
|||
While the ''[[weight]]'' of matter is entirely dependent upon the strength of local gravity, the ''mass'' of matter is constant (assuming it is not traveling at a [[Special relativity|relativistic]] speed with respect to an observer). Accordingly, for astronauts in [[Micro-g environment|microgravity]], no effort is required to hold objects off the cabin floor; they are “weightless.” However, since objects in microgravity still retain their mass, an astronaut must exert ten times as much force to ''accelerate'' a 10‑kilogram object at the same rate as a 1‑kilogram object. |
|||
The kilogram is defined in terms of three defining constants:<ref name="SIBrochure9thEd">{{citation |title=The International System of Units (SI) |author=International Bureau of Weights and Measures |author-link=New SI |date=20 May 2019 |edition=9th |isbn=978-92-822-2272-0 |url=https://www.bipm.org/utils/common/pdf/si-brochure/SI-Brochure-9.pdf| archive-url = https://web.archive.org/web/20211018184555/https://www.bipm.org/documents/20126/41483022/SI-Brochure-9.pdf/fcf090b2-04e6-88cc-1149-c3e029ad8232 |archive-date=18 October 2021 |url-status=live}}</ref> |
|||
* a specific atomic transition frequency {{math|[[ΔνCs|Δ''ν''<sub>Cs</sub>]]}}, which defines the duration of the second, |
|||
* the [[speed of light]] {{mvar|c}}, which when combined with the second, defines the length of the metre, |
|||
* and the [[Planck constant]] {{mvar|h}}, which when combined with the metre and second, defines the mass of the kilogram. |
|||
The formal definition according to the [[General Conference on Weights and Measures]] (CGPM) is: |
|||
<!-- this is an exact quote. Do not change it.-->{{Blockquote|The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the [[Planck constant]] {{mvar|h}} to be {{val|6.62607015|e=-34}} when expressed in the unit J⋅s, which is equal to kg⋅m<sup>2</sup>⋅s<sup>−1</sup>, where the [[metre]] and the [[second]] are defined in terms of {{mvar|c}} and {{math|Δ''ν''<sub>Cs</sub>}}.|source= CGPM <ref name="draft-resolution-A">{{citation |
|||
|title=Draft Resolution A "On the revision of the International System of units (SI)" to be submitted to the CGPM at its 26th meeting (2018) |
|||
|url=https://www.bipm.org/utils/en/pdf/CGPM/Draft-Resolution-A-EN.pdf |
|||
|archive-url=https://web.archive.org/web/20210402142630/https://www.bipm.org/utils/en/pdf/CGPM/Draft-Resolution-A-EN.pdf |
|||
|archive-date=April 2, 2021 |
|||
|url-status=live |
|||
}}</ref><ref>[http://www.bipm.org/en/committees/cipm/meeting/105.html Decision CIPM/105-13 (October 2016)]. The day is the 144th anniversary of the [[Metre Convention]].</ref>}} |
|||
Defined in term of those units, the kg is formulated as:<ref>[https://www.bipm.org/en/publications/si-brochure/ SI Brochure: The International System of Units (SI)]. BIPM, 9th edition, 2019.</ref> |
|||
:kg = {{math|{{sfrac|({{val|299792458}}){{sup|2}}|({{val|6.62607015|e=-34}})({{val|9192631770}})}}{{sfrac|{{gaps|''h''|Δ''ν''<sub>Cs</sub>}}|''c''{{sup|2}}}}}} = {{math|{{sfrac|{{val|917097121160018}}|{{val|62154105072590475}}}}{{val||e=42}}{{sfrac|{{gaps|''h''|Δ''ν''<sub>Cs</sub>}}|''c''{{sup|2}}}}}} ≈ {{math|({{val|1.475521399735270|e=40}}){{sfrac|{{gaps|''h''|Δ''ν''<sub>Cs</sub>}}|''c''{{sup|2}}}}}} . |
|||
This definition is generally consistent with previous definitions: the [[Mass versus weight|mass]] remains within 30 [[parts per million|ppm]] of the mass of one litre of water.<ref>The density of water is {{val|0.999972|u=g/cm3}} at {{val|3.984|u=°C}}. See {{cite book |last=Franks |first=Felix |title=The Physics and Physical Chemistry of Water |url=https://books.google.com/books?id=5f_xBwAAQBAJ&pg=PA376 |year=2012 |publisher=Springer |isbn=978-1-4684-8334-5}}</ref> |
|||
== History == |
|||
====Early definitions==== |
|||
:''See also [[Grave (mass)]] for more on the history of the kilogram.'' |
|||
=== Timeline of previous definitions === |
|||
On 7 April 1795, the [[gram]] was decreed in France to be equal to “the absolute weight of a volume of water equal to the cube of the hundredth part of the meter, at the temperature of melting ice.”<span style="margin-left:0.1em"><ref>{{cite web |
|||
[[File:International prototype of the kilogram aka Le Grand K.jpg|thumb|right|The [[International Prototype of the Kilogram]], whose mass was defined to be one kilogram from 1889 to 2019.]] |
|||
|url=http://smdsi.quartier-rural.org/histoire/18germ_3.htm |
|||
* 1793: The [[grave (unit)|grave]] (the precursor of the kilogram) was defined as the mass of 1 [[litre]] (dm<sup>3</sup>) of water, which was determined to be 18841 [[Grain (unit)|grains]].<ref>{{cite book |title=Annales de chimie ou Recueil de mémoires concernant la chimie et les arts qui en dépendent |date=1792|url=https://books.google.com/books?id=FufDNJHvgFEC&q=18841+grains+grave&pg=RA1-PA278 |location= Paris |publisher= Chez Joseph de Boffe |page= 277 |volume = 15–16| last1=Guyton| last2=Lavoisier| last3=Monge| last4=Berthollet| display-authors=etal|author-link1=Louis-Bernard Guyton de Morveau| author-link2=Antoine Lavoisier| author-link3=Gaspard Monge| author-link4=Claude Louis Berthollet}}</ref> |
|||
|title=Decree on weights and measures |
|||
* 1795: the gram (<sup>1</sup>/<sub>1000</sub> of a kilogram) was provisionally defined as the mass of one cubic [[centimetre]] of water at the melting point of ice.<ref>{{lang|fr|Gramme, le poids absolu d'un volume d'eau pure égal au cube de la centième partie du mètre, et à la température de la glace fondante}}</ref> |
|||
|date=7 April 1795 |
|||
* 1799: The [[Kilogramme des Archives]] was manufactured as a prototype. It had a mass equal to the mass of 1 dm<sup>3</sup> of water at the temperature of its maximum density, which is approximately 4 [[Celsius|°C]].<ref name="Zupko">{{cite book |last=Zupko |first=Ronald Edward| author-link =Ronald Edward Zupko|date=1990 |title=Revolution in Measurement: Western European Weights and Measures Since the Age of Science |url=https://archive.org/details/bub_gb_uYCNFkRgXCoC |location=Philadelphia |publisher=American Philosophical Society |isbn=978-0-87169-186-6}}</ref> |
|||
|quotation=''Gramme'', le poids absolu d'un volume d'eau pure égal au cube de la centième partie du mètre , et à la température de la glace fondante. |
|||
* 1875–1889: The [[Metre Convention]] was signed in 1875, leading to the production of the [[International Prototype of the Kilogram]] (IPK) in 1879 and its adoption in 1889.<ref>{{cite encyclopedia|url = http://www.britannica.com/EBchecked/topic/378767/Treaty-of-the-Metre|title = Treaty of the Metre|encyclopedia = [[Encyclopædia Britannica]]|access-date = 18 July 2023|year = 2023}}</ref> |
|||
}}</ref></span> Since trade and commerce typically involve items significantly more massive than one gram, and since a mass standard made of water would be inconvenient and unstable, the regulation of commerce necessitated the manufacture of a ''[[#Glossary|practical realization]]'' of the water-based [[#Glossary|definition]] of mass. Accordingly, a provisional mass standard was made as a single-piece, metallic [[#Glossary|artifact]] one thousand times more massive than the gram—the kilogram. |
|||
* 2019: The kilogram was [[2019 redefinition of the SI base units|defined]] in terms of the [[Planck constant]], the [[speed of light]] and [[Caesium standard|hyperfine transition frequency of <sup>133</sup>Cs]] as approved by the [[General Conference on Weights and Measures]] (CGPM) on November 16, 2018.<ref name="vox"/> |
|||
== Name and terminology == |
|||
At the same time, work was commissioned to precisely determine the mass of a cubic decimeter (one [[litre|liter]]<span style="margin-left:0.2em"><ref> |
|||
The kilogram is the only [[SI base unit|base SI unit]] with an [[SI prefix]] (''kilo'') as part of its name. The word ''kilogramme'' or ''kilogram'' is derived from the [[French language|French]] {{lang|fr|kilogramme}},<ref name=OED/> which itself was a learned coinage, prefixing the [[Koine Greek|Greek]] stem of {{lang|grc|χίλιοι}} {{transl|grc|khilioi}} "a thousand" to {{lang|la|gramma}}, a Late Latin term for "a small weight", itself from Greek {{lang|grc|γράμμα}}.<ref> |
|||
The same decree {{cite web |
|||
{{cite book |
|||
|url=http://smdsi.quartier-rural.org/histoire/18germ_3.htm |
|||
|title = The Concise Oxford Dictionary |
|||
|year = 1964 |
|||
|date=7 April 1795 |
|||
|first1 = HW |
|||
}} also defined the liter as “the measure of volume, both for liquid and solids, for which the displacement would be that of a cube [with sides measuring] one-tenth of a meter.” Original text: “''Litre'', la mesure de capacité, tant pour les liquides que pour les matières sèches, dont la contenance sera celle du cube de la dixièrne partie du mètre.”</ref></span>) of water. Although the decreed definition of the kilogram specified water at 0 °C—its highly stable ''temperature'' point—the French chemist, Louis Lefèvre‑Gineau<ref>[http://translate.google.com/translate?u=http%3A%2F%2Ffr.wikipedia.org%2Fwiki%2FLouis_Lefèvre-Gineau&sl=fr&tl=en&hl=en&ie=UTF-8 Google translation of {{nowrap|fr.Wikipedia}} article on Louis Lefèvre‑Gineau]</ref> and the Italian naturalist, Giovanni Valentino Fabbroni<ref>[http://translate.google.com/translate?hl=en&sl=it&u=http://it.wikipedia.org/wiki/Giovanni_Fabbroni&sa=X&oi=translate&resnum=3&ct=result&prev=/search%3Fq%3DGiovanni%2BFabbroni%2BWikipedia%26hl%3Den%26client%3Dsafari%26rls%3Den-us%26sa%3DG%26pwst%3D1 Google translation of {{nowrap|it.Wikipedia}} article on Giovanni Fabbroni]</ref> after several years of research chose to redefine the standard in 1799 to water’s most stable ''density'' point: the temperature at which water reaches maximum density, which was measured at the time as 4 °C.<ref>Modern measurements show the temperature at which water reaches maximum density is 3.984 °C. However, the scientists at the close of the 18th century concluded that the temperature was 4 °C. {{cite web |
|||
|last1 = Fowlers |
|||
|title=L'histoire du mètre, la détermination de l'unité de poids |
|||
|first2 = FG |
|||
|url=http://histoire.du.metre.free.fr/fr/index.htm |
|||
|last2 = Fowler |
|||
}}</ref> They concluded that one cubic decimeter of water at its maximum density was equal to 99.9265% of the target mass of the provisional kilogram standard made four years earlier.<!-- |
|||
|publisher = The Clarendon Press |
|||
|location = Oxford |
|||
}} Greek {{lang|grc|γράμμα}} (as it were {{lang|grc|[[:wikt:γράφω|γράφ]]-[[:wikt:-μα|μα]]}}, Doric {{lang|grc|γράθμα}}) means "something written, a letter", but it came to be used as a unit of weight, apparently equal to {{sfrac|1|24}} of an [[ounce]] ({{sfrac|1|288}} of a {{lang|la|[[Pound (mass)#Roman libra|libra]]}}, which would correspond to about 1.14 grams in modern units), at some time during Late Antiquity. French {{lang|fr|gramme}} was adopted from Latin {{lang|la|gramma}}, itself quite obscure, but found in the {{lang|la|Carmen de ponderibus et mensuris}} (8.25) attributed by [[Remmius Palaemon]] (fl. 1st century), where it is the weight of two {{lang|la|[[obolus|oboli]]}} (Charlton T. Lewis, Charles Short, ''A Latin Dictionary'' [https://www.perseus.tufts.edu/hopper/text?doc=Perseus%3Atext%3A1999.04.0059%3Aentry%3Dgramma2 s.v. "gramma"], 1879). |
|||
Henry George Liddell. Robert Scott. ''[[A Greek-English Lexicon]]'' (revised and augmented edition, Oxford, 1940) [https://www.perseus.tufts.edu/hopper/text?doc=Perseus:text:1999.04.0057:entry=gra/mma s.v. γράμμα], citing the 10th-century work ''[[Geoponica]]'' and a 4th-century papyrus edited in L. Mitteis, ''Griechische Urkunden der Papyrussammlung zu Leipzig'', vol. i (1906), 62 ii 27.</ref> |
|||
The word {{lang|fr|kilogramme}} was written into French law in 1795, in the ''Decree of [[French Republican Calendar|18 Germinal]]'',<ref> |
|||
{{cite web |
|||
|url = http://mjp.univ-perp.fr/france/1793mesures.htm |
|||
|title = Décret relatif aux poids et aux mesures du 18 germinal an 3 (7 avril 1795) |
|||
|language = fr |
|||
|trans-title=Decree of 18 Germinal, year III (April 7, 1795) regarding weights and measures |
|||
|website = Grandes lois de la République |
|||
|publisher = Digithèque de matériaux juridiques et politiques, Université de Perpignan |
|||
|access-date = November 3, 2011 |
|||
}}</ref> |
|||
which revised the provisional system of units introduced by the French [[National Convention]] two years earlier, where the {{lang|fr|gravet}} had been defined as weight ({{lang|fr|poids}}) of a cubic centimetre of water, equal to 1/1000 of a {{lang|fr|[[Grave (unit)|grave]]}}.<ref>{{lang|fr|Convention nationale, décret du 1<sup>er</sup> août 1793, ed. Duvergier, ''Collection complète des lois, décrets, ordonnances, règlemens avis du Conseil d'état, publiée sur les éditions officielles du Louvre''|italic=unset}}, vol. 6 (2nd ed. 1834), [https://books.google.com/books?id=0mYZAAAAYAAJ&pg=PA70 p. 70]. |
|||
The ''metre'' ({{lang|fr|mètre}}) on which this definition depends was itself defined as the ten-millionth part of a quarter of Earth's [[Meridian (geography)|meridian]], given in [[Units of measurement in France before the French Revolution|traditional units]] as 3 {{lang|fr|[[foot (unit)|pieds]]}}, 11.44 {{lang|fr|lignes}} (a {{lang|fr|ligne}} being the 12th part of a {{lang|fr|pouce}} (inch), or the 144th part of a {{lang|fr|pied}}.</ref> In the decree of 1795, the term {{lang|fr|gramme}} thus replaced {{lang|fr|gravet}}, and {{lang|fr|kilogramme}} replaced {{lang|fr|grave}}.<ref name="Zupko"/> |
|||
The French spelling was adopted in Great Britain when the word was used for the first time in English in 1795,<ref>{{Cite journal |url = https://books.google.com/books?id=24QCAAAAYAAJ&q=kilogramme+weights&pg=PA557 |
|||
--><ref>The provisional kilogram standard had been fabricated in accordance with a single, inaccurate measurement of the density of water made earlier by [[Antoine Lavoisier]] and [[René Just Haüy]], which showed that one cubic decimeter of distilled water at 0 °C had a mass of 18,841 grains<!--EDITORS NOTE: DO NOT LINK “grains” to [[Grain (mass)]] because the poids de marc grain is a different unit. --> in France’s soon-to-be-obsoleted ''poids de marc'' system. The newer, highly accurate measurements by Lefèvre‑Gineau and Fabbroni concluded that the mass of a cubic decimeter of water at the new temperature of 4 °C—a condition at which water is denser—was actually ''less massive'', at 18,827.15 grains, than the earlier inaccurate value assumed for 0 °C water.<p><!-- |
|||
|title = Paris, during the year 1795 |
|||
|author = Peltier, Jean-Gabriel |journal = Monthly Review |date=1795 |volume=17|pages=556|access-date = August 2, 2018 |
|||
}} Contemporaneous English translation of the French decree of 1795</ref><ref name=OED>{{cite web |
|||
|url = http://www.oed.com/viewdictionaryentry:showfullentry/true?t:ac=Entry/103396 |
|||
|website = Oxford English Dictionary |
|||
|publisher = Oxford University Press |
|||
|title = Kilogram |
|||
|access-date = November 3, 2011}}</ref> with the spelling ''kilogram'' being adopted in the United States. In the United Kingdom both spellings are used, with "kilogram" having become by far the more common.<ref name=":1"> |
|||
{{cite web |
|||
|url = http://english.oxforddictionaries.com/definition/kilogram |
|||
|title = Kilogram |
|||
|website = Oxford Dictionaries |
|||
|access-date = November 3, 2011 |
|||
|url-status = dead |
|||
|archive-url = https://archive.today/20130131014115/http://english.oxforddictionaries.com/definition/kilogram |
|||
|archive-date = January 31, 2013 |
|||
|df = mdy-all |
|||
}}</ref> UK law regulating the units to be used when [[Weights and Measures Acts of the United Kingdom|trading by weight or measure]] does not prevent the use of either spelling.<ref> |
|||
{{cite web |
|||
|url = http://www.legislation.gov.uk/ukpga/1985/72/section/92 |
|||
|title = Spelling of "gram", etc |
|||
|website = [[Weights and Measures Acts of the United Kingdom|Weights and Measures Act 1985]] |
|||
|publisher = [[Office of Public Sector Information|Her Majesty's Stationery Office]] |
|||
|date = October 30, 1985 |
|||
|access-date = November 6, 2011 |
|||
}}</ref> |
|||
In the 19th century the French word {{lang|fr|kilo}}, a [[Clipping (morphology)|shortening]] of {{lang|fr|kilogramme}}, was imported into the English language where it has been used to mean both kilogram<ref> |
|||
-->France’s metric system had been championed by [[Charles Maurice de Talleyrand-Périgord|Charles Maurice de Talleyrand‑Périgord]]. On 30 March 1791, four days after Talleyrand forwarded a specific proposal on how to proceed with the project, the French government ordered a committee known as the Academy to commence work on accurately determining the magnitude of the base units of the new metric system. The Academy divided the task among five commissions. The commission charged with determining the mass of a cubic decimeter of water originally comprised Lavoisier and Haüy but their work was finished by Louis Lefèvre‑Gineau and Giovanni Fabbroni.<p><!-- |
|||
{{cite encyclopedia |
|||
|year=1989 |
|||
|edition = 2nd |
|||
|title = kilo (n1) |
|||
|encyclopedia = [[Oxford English Dictionary]] |
|||
|publisher = Oxford University Press |
|||
|location = Oxford |
|||
|url = http://www.oed.com/viewdictionaryentry/Entry/103394 |
|||
|access-date = November 8, 2011}}</ref> and kilometre{{fact|date=December 2023}}.<ref>{{cite encyclopedia |
|||
|year = 1989 |
|||
|edition = 2nd |
|||
|title = kilo (n2) |
|||
|encyclopedia = [[Oxford English Dictionary]] |
|||
|publisher = Oxford University Press |
|||
|location = Oxford |
|||
|url = http://www.oed.com/viewdictionaryentry/Entry/103395 |
|||
|access-date = November 8, 2011 |
|||
}}</ref> While ''kilo'' as an alternative is acceptable, to ''[[The Economist]]'' for example,<ref>{{cite news |url=http://www.frzee.com/Education/The%20Economist%20Style%20Guide.pdf |title=Style Guide |newspaper=[[The Economist]] |date=January 7, 2002 |archive-url=https://web.archive.org/web/20170701053545/http://www.frzee.com/Education/The%20Economist%20Style%20Guide.pdf |archive-date=July 1, 2017 |url-status=dead |access-date=November 8, 2011}}</ref> the Canadian government's [[Termium Plus]] system states that "SI (International System of Units) usage, followed in scientific and technical writing" does not allow its usage and it is described as "a common informal name" on Russ Rowlett's Dictionary of Units of Measurement.<ref> |
|||
{{cite web |url=https://www.btb.termiumplus.gc.ca/tpv2guides/guides/wrtps/index-eng.html?lang=eng&lettr=indx_catlog_k&page=96vUJlKx4UCA.html |website=Termium Plus |publisher=Government of Canada |title=kilogram, kg, kilo |date=October 8, 2009 |access-date =May 29, 2019 }}</ref><ref> |
|||
{{cite web |url=http://www.unc.edu/~rowlett/units/dictK.html |title=kilo |website=How Many? |access-date=November 6, 2011 |url-status=dead |archive-url=https://web.archive.org/web/20111116205434/http://www.unc.edu/~rowlett/units/dictK.html |archive-date=November 16, 2011}}</ref> When the [[United States Congress]] gave the metric system legal status in 1866, it permitted the use of the word ''kilo'' as an alternative to the word ''kilogram'',<ref> |
|||
{{cite web |
|||
|url=http://lamar.colostate.edu/~hillger/laws/metric-act-bill.html |
|||
|title=H.R. 596, An Act to authorize the use of the metric system of weights and measures |
|||
|author=29th Congress of the United States, Session 1 |
|||
|date=May 13, 1866 |
|||
|url-status=dead |
|||
|archive-url=https://web.archive.org/web/20150705015307/http://lamar.colostate.edu/~hillger/laws/metric-act-bill.html |
|||
|archive-date=July 5, 2015 |
|||
}}</ref> but in 1990 revoked the status of the word ''kilo''.<ref> |
|||
{{cite journal |
|||
|journal = [[Federal Register]] |
|||
|volume = 63 |
|||
|issue = 144 |
|||
|date = July 28, 1998 |
|||
|page = 40340 |
|||
|url = http://physics.nist.gov/cuu/pdf/SIFedReg.pdf |
|||
|title = Metric System of Measurement:Interpretation of the International System of Units for the United States; Notice |
|||
|quote = '''Obsolete Units''' As stated in the 1990 Federal Register notice, ... |
|||
|access-date = November 10, 2011 |
|||
|url-status = dead |
|||
|archive-url = https://web.archive.org/web/20111015081850/http://physics.nist.gov/cuu/pdf/SIFedReg.pdf |
|||
|archive-date = October 15, 2011 |
|||
|df = mdy-all |
|||
}}</ref> |
|||
The SI system was introduced in 1960 and in 1970 the [[International Bureau of Weights and Measures|BIPM]] started publishing the [[International System of Units#SI Brochure|''SI Brochure'']], which contains all relevant decisions and recommendations by the [[General Conference on Weights and Measures|CGPM]] concerning units. The ''SI Brochure'' states that "It is not permissible to use abbreviations for unit symbols or unit names ...".<ref>{{SIBrochure8th|page = 130}}</ref><ref group = Note>The French text (which is the authoritative text) states "{{lang|fr|Il n'est pas autorisé d'utiliser des abréviations pour les symboles et noms d'unités ...}}"</ref> |
|||
-->Neither Lavoisier nor Haüy can be blamed for participating in an initial—and inaccurate—measurement and for leaving the final work to Lefèvre‑Gineau and Fabbroni to finish in 1799. As a member of the ''[[Ferme générale]]'', Lavoisier was also one of France’s 28 tax collectors. He was consequently convicted of treason during the waning days of of the [[Reign of Terror]] period of the [[French Revolution]] and beheaded on 8 May 1794. Lavoisier’s partner, Haüy, was also thrown into prison and was himself at risk of going to the guillotine but his life was spared after a renown French naturalist interceded.<p>{{cite book |
|||
|author=Ronald Edward Zupko |
|||
|title=Revolution in Measurement: Western European Weights and Measures Since the Age of Science |
|||
|publisher=DIANE Publishing |
|||
|year=1990 |
|||
|isbn= |
|||
}}</ref><!-- |
|||
For use with east Asian character sets, the SI symbol is encoded as a single Unicode character, {{unichar|338f|SQUARE KG}} in the [[CJK Compatibility]] block. |
|||
--> That same year, 1799, an all-platinum kilogram [[#Glossary|prototype]] was fabricated with the objective that it would equal, as close as was scientifically feasible for the day, the mass of one cubic decimeter of water at 4 °C. The prototype was presented to the Archives of the Republic in June and on 10 December 1799, the prototype was formally ratified as the ''Kilogramme des Archives'' (Kilogram of the Archives) and the kilogram was defined as being equal to its mass. This standard stood for the next ninety years. |
|||
== Redefinition based on fundamental constants == |
|||
====International Prototype Kilogram==== |
|||
[[File:Unit relations in the new SI.svg|thumb|upright=1.35|The [[International System of Units|SI system]] after the 2019 redefinition: the kilogram is now fixed in terms of the [[second]], the [[speed of light]] and the [[Planck constant]]; furthermore the [[ampere]] no longer depends on the kilogram]] |
|||
<!--NOTE TO EDITORS: This section is externally linked to from [[Specific heat capacity]] and [[Mass versus weight]]. Please do not delete or rename without fixing the referencing link.--> |
|||
[[File:Watt balance, large view.jpg|thumb|A [[Kibble balance]], which was originally used to measure the [[Planck constant]] in terms of the IPK, can now be used to calibrate secondary standard weights for practical use.]] |
|||
Since 1889, the [[International System of Units|SI]] system defines the [[#Glossary|magnitude]] of the kilogram to be equal to the mass of the ''International Prototype Kilogram'',<ref name="FirstCGPM"/> often referred to in the professional [[metrology]] world as the “IPK”. The IPK is made of a platinum [[alloy]] known as “Pt‑10Ir”, which is 90% [[platinum]] and 10% [[iridium]] (by weight) and is machined into a right-circular cylinder (height = diameter) of 39.17 [[Millimetre|mm]] to minimize its surface area.<ref name="Quinn">''New Techniques in the Manufacture of Platinum-Iridium Mass Standards'', T. J. Quinn, Platinum Metals Rev., 1986, '''30''', (2), pp. {{nowrap|74–79}}<sub> </sub></ref> The addition of 10% iridium improved upon the all-platinum Kilogram of the Archives by greatly increasing [[hardness]] while still retaining platinum’s many virtues: extreme resistance to [[Redox|oxidation]], extremely high [[density]], satisfactory [[Electrical conductivity|electrical]] and [[Thermal conductivity|thermal conductivities]], and low [[magnetic susceptibility]]. The IPK and its six [[#Glossary|sister copies]] are stored at the [[International Bureau of Weights and Measures]] (known by its French-language initials BIPM) in an environmentally monitored safe in the lower vault located in the basement of the BIPM’s House of Breteuil in [[Sèvres]] on the outskirts of Paris (see ''[[#External links|External links]]'', below for images). Three independently controlled keys are required to open the vault. Official copies of the IPK were made available to other nations to serve as their national standards. These are compared to the IPK roughly every 50 years. |
|||
{{main|2019 redefinition of the SI base units}} |
|||
The replacement of the [[International Prototype of the Kilogram]] (IPK) as the primary standard was motivated by evidence accumulated over a long period of time that the mass of the IPK and its replicas had been changing; the IPK had diverged from its replicas by approximately 50 micrograms since their manufacture late in the 19th century. This led to [[Alternative approaches to redefining the kilogram|several competing efforts]] to develop measurement technology precise enough to warrant replacing the kilogram artefact with a definition based directly on physical fundamental constants.<ref name="vox">{{cite news |last1=Resnick |first1=Brian |title=The new kilogram just debuted. It's a massive achievement. |url=https://www.vox.com/science-and-health/2019/5/17/18627757/kilogram-redefined-world-metrology-day-explained |access-date=May 23, 2019 |publisher=vox.com |date=May 20, 2019}}</ref> |
|||
The IPK is one of three cylinders made in 1879. In 1883, it was found to be indistinguishable from the mass of the Kilogram of the Archives made eighty-four years prior, and was formally ratified as ''the'' kilogram by the 1st [[General Conference on Weights and Measures|CGPM]] in 1889.<ref name="Quinn"/> Modern measurements of the density of [[Vienna Standard Mean Ocean Water]]—purified water that has a carefully controlled isotopic composition—show that a cubic decimeter of water at its point of maximum density, 3.984 °C, has a mass that is 25.05 [[Parts-per notation|parts per million]] less than the kilogram.<ref>''Water Structure and Science, Water Properties, Density maximum (and molar volume) at temperature of maximum density, a'' (by London South Bank University). [http://www.lsbu.ac.uk/water/data.html Link to Web site.]<sub> </sub></ref> This small difference, and the fact that the mass of the IPK was indistinguishable from the mass of the Kilogram of the Archives, speak volumes of the scientists’ skills over {{age|1799|1|1}} years ago when making their measurements of water’s properties and in manufacturing the Kilogram of the Archives. |
|||
The [[International Committee for Weights and Measures]] (CIPM) approved a [[2019 redefinition of the SI base units|redefinition of the SI base units]] in November 2018 that defines the kilogram by defining the [[Planck constant]] to be exactly {{val|6.62607015|e=−34|u=kg⋅m<sup>2</sup>⋅s<sup>−1</sup>}}, effectively defining the kilogram in terms of the second and the metre. The new definition took effect on May 20, 2019.<ref name="vox"/><ref name=draft-resolution-A /><ref> |
|||
==Stability of the International Prototype Kilogram== |
|||
{{cite news |
|||
[[Image:Prototype mass drifts.jpg|thumb|right|399px|Mass drift over time of national prototypes {{nowrap|K21–K40}}, plus two of the IPK’s [[#Glossary|sister copies]]: K8(41)<ref>Prototype No. 8(41) was accidentally stamped with the number 41, but its accessories carry the proper number 8. Since there is no prototype marked 8, this prototype is referred to as 8(41).<sub> </sub></ref> and K32. All mass changes are relative to the IPK. The initial 1889 starting-value offsets relative to the IPK have been nulled.<ref name="Girard">''The Third Periodic Verification of National Prototypes of the Kilogram {{nowrap|(1988–1992)'',}} G. Girard, Metrologia '''31''' (1994) {{nowrap|317–336}}<sub> </sub></ref> The above are all ''relative'' measurements; no historical mass-measurement data is available to determine which of the prototypes has been most stable relative to an invariant of nature. There is the distinct possibility that ''all'' the prototypes gained mass over 100 years and that K21, K35, K40, and the IPK simply ''gained less'' than the others.]]<!-- |
|||
|url= https://www.bbc.com/news/science-environment-46143399 |
|||
|title= Kilogram gets a new definition |
|||
|author= Pallab Ghosh |
|||
|date= November 16, 2018 |
|||
|journal= BBC News |
|||
|access-date= November 16, 2018 |
|||
}}</ref> |
|||
Prior to the redefinition, the kilogram and several other SI units based on the kilogram were defined by a man-made metal artifact: the ''[[Kilogramme des Archives]]'' from 1799 to 1889, and the IPK from 1889 to 2019.<ref name="vox"/> |
|||
-->By definition, the error in the measured value of the [[#Glossary|IPK]]’s mass is exactly zero; the IPK ''is'' the kilogram. However, any changes in the IPK’s mass over time can be deduced by comparing its mass to that of its official copies stored throughout the world, a process called “periodic verification.” For instance, the U.S. owns four {{nowrap|90% platinum / 10% iridium}} (Pt‑10Ir) kilogram standards, two of which, K4 and K20, are from the original batch of 40 [[#Glossary|replicas]] delivered in 1884.<ref>The other two Pt‑10Ir standards owned by the U.S. are K85, which is used for watt balance experiments (see ''[[#Watt_balance|Watt balance]],'' above), and K650, which was an early attempt with a new series of prototypes {{nowrap|(K64–K80)}} that were diamond-turned directly to a finish mass. K650 has an unremarkable density of {{val|21.53537|u=g/ml}}. However, as its finished mass was roughly 2000 µg less than one kilogram, it is unsuitable for use as a national prototype and is instead known as a mass “standard”—not a “prototype.”. However, it serves well for special duties, such as a stability reference when K4 and K20 are transported to the BIPM and back. There are three other diamond-turned, Pt‑10Ir replicas that are not formally considered to be “prototypes”: K651, K690, and K691.<sub> </sub></ref> The K20 prototype was designated as the [[#Glossary|primary national standard]] of mass for the U.S. Both of these, as well as those from other nations, are periodically returned to the BIPM for verification.<ref>Extraordinary care is exercised when transporting prototypes. In 1984, the K4 and K20 prototypes were hand-carried in the passenger section of a commercial airliner.<sub> </sub></ref> |
|||
In 1960, the [[metre]], previously similarly having been defined with reference to a single platinum-iridium bar with two marks on it, was redefined in terms of an invariant physical constant (the wavelength of a particular emission of light emitted by [[krypton]],<ref>{{SIbrochure8th|page=112}}</ref> and later the [[speed of light]]) so that the standard can be independently reproduced in different laboratories by following a written specification. |
|||
Note that none of the replicas has a mass precisely equal to that of the IPK<!-- the closest is Hungary’s K16 at 1 kg +12 µg (Girard) -->; their masses are calibrated and documented as offset values. For instance, K20, the U.S.’s primary standard, originally had an official mass of {{nowrap|1 kg − 39 µg}} in 1889; that is to say, K20 was 39 µg less than the IPK. A verification performed in 1948 showed a mass of {{nowrap|1 kg − 19 µg.}} The latest verification performed in 1999 shows a mass precisely identical to its original 1889 value. Quite unlike transient variations such as this, the U.S.’s [[#Glossary|check standard]], K4, has persistently declined in mass relative to the IPK—and for an identifiable reason. Check standards are used much more often than primary standards and are prone to scratches and other wear. K4 was originally delivered with an official mass of {{nowrap|1 kg − 75 µg}} in 1889, but as of 1989 was officially calibrated at {{nowrap|1 kg − 106 µg}} and ten years later was {{nowrap|1 kg − 116 µg.}} Over a period of 110 years, K4 lost 41 µg relative to the IPK.<ref name="Recalibration">''The Kilogram and Measurements of Mass and Force'', Z. J. Jabbour ''et al.'', J. Res. Natl. Inst. Stand. Technol. '''106''', 2001, {{nowrap|25–46}} ([http://nvl.nist.gov/pub/nistpubs/jres/106/1/j61jab.pdf 3.5 MB PDF, here])<sub> </sub></ref> |
|||
At the 94th Meeting of the CIPM in 2005, it was recommended that the same be done with the kilogram.<ref> |
|||
Beyond the simple wear that check standards can experience, the mass of even the carefully stored national prototypes can drift relative to the IPK for a variety of reasons, some known and some unknown. Since the IPK and its replicas are stored in air (albeit under two or more nested [[bell jar]]s), they gain mass through [[adsorption]] of atmospheric contamination onto their surfaces. Accordingly, they are cleaned in a process the BIPM developed between 1939 and 1946 known as “the BIPM cleaning method” that comprises lightly rubbing with a [[Chamois leather|chamois]] soaked in equal parts [[ether]] and [[ethanol]], [[steam cleaning]] with bi-[[Distillation|distilled]] water, and allowing the [[#Glossary|prototypes]] to settle for {{nowrap|7–10}} days before verification.<ref>Before the BIPM’s published report in 1994 detailing the relative change in mass of the prototypes, different standard bodies used different techniques to clean their prototypes. The NIST’s practice before then was to soak and rinse its two prototypes first in [[benzene]], then in ethanol, and to then clean them with a jet of bi-distilled water steam.<sub> </sub></ref> Cleaning the prototypes removes between 5 and 60 µg of contamination depending largely on the time elapsed since the last cleaning. Further, a second cleaning can remove up to 10 µg more. After cleaning—even when they are stored under their bell jars—the IPK and its replicas immediately begin gaining mass again. The BIPM even developed a model of this gain and concluded that it averaged 1.11 µg per month for the first 3 months after cleaning and then decreased to an average of about 1 µg per year thereafter. Since check standards like K4 are not cleaned for routine calibrations of other mass standards—a precaution to minimize the potential for wear and handling damage—the BIPM’s model has been used as an “after cleaning” correction factor. |
|||
{{cite conference |
|||
|url=https://www.bipm.org/cc/CIPM/Allowed/94/CIPM-Recom1CI-2005-EN.pdf |
|||
|conference=94th meeting of the International Committee for Weights and Measures |
|||
|title=Recommendation 1: Preparative steps towards new definitions of the kilogram, the ampere, the kelvin and the mole in terms of fundamental constants |
|||
|date=October 2005 |
|||
|page=233 |
|||
|access-date=February 7, 2018 |
|||
|url-status=live |
|||
|archive-url=https://web.archive.org/web/20070630011658/https://www.bipm.org/cc/CIPM/Allowed/94/CIPM-Recom1CI-2005-EN.pdf |
|||
|archive-date=June 30, 2007 |
|||
}}</ref><!-- Original URL: http://www.bipm.org/utils/en/pdf/CIPM2005-EN.pdf --> |
|||
In October 2010, the CIPM voted to submit a resolution for consideration at the [[General Conference on Weights and Measures]] (CGPM), to "take note of an intention" that the kilogram be defined in terms of the [[Planck constant]], {{math|''h''}} (which has dimensions of energy times time, thus mass × length{{sup|2}} / time) together with other physical constants.<ref>{{cite journal|url=https://www.nist.gov/pml/wmd/20101026_si.cfm |title=NIST Backs Proposal for a Revamped System of Measurement Units |journal=NIST |date=October 26, 2010 |publisher=Nist.gov |access-date=April 3, 2011}}</ref><ref name="draft"> |
|||
[[Image:Denmark’s K48 Kilogram.jpg|thumb|left|234px|K48 came from the second batch of kilogram replicas to be produced. It was delivered to Denmark in 1949 with an official mass of {{nowrap|1 kg + 81 µg.}} Like all other replicas, it is stored under two nested bell jars virtually all the time. Still, its mass and that of the IPK diverged markedly in only 40 years; the mass of K48 was certified as {{nowrap|1 kg + 112 µg}} during the {{nowrap|1988–1992}} periodic verification.<ref name="Girard"/>]]<!-- |
|||
-->Because the first forty official copies are made of the same alloy as the IPK and are stored under similar conditions, periodic verifications using a large number of replicas—especially the national primary standards, which are rarely used—can convincingly demonstrate the stability of the IPK. What has become clear after the third periodic verification performed between 1988 and 1992 is that masses of the entire worldwide ensemble of prototypes have been slowly but inexorably diverging from each other. It is also clear that the mass of the IPK lost perhaps 50 µg over the last century, and possibly significantly more, in comparison to its official copies.<!-- |
|||
--><ref name="Girard"/><ref name="Redef">''Redefinition of the kilogram: a decision whose time has come'', Ian M. Mills ''et al.'', Metrologia '''42''' (2005), {{nowrap|71–80}}<sub> </sub></ref> <!-- |
|||
-->The reason for this drift has eluded physicists who have dedicated their careers to the SI unit of mass. No plausible mechanism has been proposed to explain either a steady decrease in the mass of the IPK, or an increase in that of its replicas dispersed throughout the world.<!-- |
|||
--><ref>Citations: ''The SI unit of mass'', Richard Davis, Metrologia '''40''' (2003), {{nowrap|299–305;}} and ''Recalibration of the U.S. National Prototype Kilogram'', R. S. Davis, Journal of Research of the National Bureau of Standards, '''90''', No. 4, {{nowrap|July–August}} 1985 ([http://nvl.nist.gov/pub/nistpubs/jres/090/4/V90-4.pdf 5.5 MB PDF, here]). Note that if the ∆ 50 µg between the IPK and its replicas was entirely due to wear, the IPK would have to have lost 150 million billion more platinum and iridium atoms over the last century than its replicas. That there would be this much wear, much less a ''difference'' of this magnitude, is thought unlikely; 50 µg is roughly the mass of a fingerprint. Specialists at the BIPM in 1946 carefully conducted cleaning experiments and concluded that even ''vigorous'' rubbing with a chamois—if done carefully—did not alter the prototypes’ mass. More recent cleaning experiments at the BIPM, which were conducted on one particular prototype (K63), and which benefited from the then-new NBS‑2 balance, demonstrated 2 µg stability.<p>Many theories have been advanced to explain the divergence in the masses of the prototypes. One theory posits that the relative change in mass between the IPK and its replicas is not one of loss at all, and is instead a simple matter that the IPK has ''gained less'' than the replicas. This theory begins with the observation that the IPK is uniquely stored under three nested bell jars whereas its six sister copies stored alongside it in the vault as well as the other replicas dispersed throughout the world are stored under only two. This theory is also founded on two other facts: that platinum has a strong affinity for mercury, and that atmospheric mercury is significantly more abundant in the atmosphere today than at the time the IPK and its replicas were manufactured. The burning of coal is a major contributor to atmospheric mercury and both Denmark and Germany have high coal shares in electrical generation. Conversely, electrical generation in France, where the IPK is stored, is mostly nuclear. This theory is supported by the fact that the mass divergence rate—relative to the IPK—of Denmark’s prototype, K48, since it took possession in 1949 is an especially high 78 µg per century while that of Germany’s prototype has been even greater at {{nowrap|126 µg/century}} ever since it took possession of K55 in 1954. However, still other data for other replicas isn’t supportive of this theory. This mercury absorption theory is just one of many advanced by the specialists to account for the relative change in mass. To date, each theory has either proven implausible, or there are insufficient data or technical means to either prove or disprove it. Citation: ''Conjecture why the IPK drifts'', R. Steiner, NIST, 11 Sept. 2007.<sub> </sub></ref> <!-- |
|||
-->This ''relative'' nature of the changes amongst the world’s kilogram prototypes is often misreported in the popular press, and even some notable scientific magazines, which often state that the IPK simply “lost 50 µg” and omit the very important caveat of ''“in comparison to its official copies''.”<span style="margin-left:0.2em"><!-- |
|||
--><ref>Even well respected organizations incorrectly represent the relative nature of the mass divergence as being one of mass loss, as exemplified by [http://www.sciencedaily.com/releases/2007/09/070921110735.htm this site at Science Daily,] and [http://www.physorg.com/news109595312.html this site at PhysOrg.com,] and [http://www.sandia.gov/LabNews/080201.html this site at Sandia National Laboratories.] The root of the problem is often the reporters’ failure to correctly interpret or paraphrase nuanced scientific concepts, as exemplified by [http://www.physorg.com/news108836759.html this 12 September 2007 story] by the [[Associated Press]] published on [[PhysOrg.com]]. In that AP story, Richard Davis—who used to be the NIST’s kilogram specialist and now works for the BIPM in France—was correctly quoted by the AP when he stated that the mass change is a relative issue. Then the AP summarized the nature of issue with this lead-in to the story: ''“A kilogram just isn't what it used to be. The 118-year-old cylinder that is the international prototype for the metric mass, kept tightly under lock and key outside Paris, is mysteriously losing weight - if ever so slightly.”'' Like many of the above-linked sites, the AP also misreported the age of the IPK, using the date of its adoption as the mass prototype, not the date of the cylinder’s manufacture. This is a mistake even [[Scientific American]] fell victim to in a print edition.<sub> </sub></ref></span> <!-- |
|||
-->Further, there is no technical means available to determine whether or not the entire worldwide ensemble of prototypes suffers from even greater long-term trends upwards or downwards because their mass “relative to an invariant of nature is unknown at a level below 1000 µg over a period of 100 or even 50 years.”<ref name="Redef"/> Given the lack of data identifying which of the world’s kilogram prototypes has been most stable in absolute terms, it is equally as valid to state that the first batch of replicas has, as a group, gained an average of about 25 µg over one hundred years in comparison to the IPK.<ref>The mean change in mass of the first batch of replicas relative to the IPK over one hundred years is +23.5 µg with a standard deviation of 30 µg. Per ''The Third Periodic Verification of National Prototypes of the Kilogram {{nowrap|(1988–1992)'',}} G. Girard, Metrologia '''31''' (1994) Pg. 323, Table 3. Data is for prototypes K1, K5, K6, K7, K8(41), K12, K16, K18, K20, K21, K24, K32, K34, K35, K36, K37, K38, and K40; and excludes K2, K23, and K39, which are treated as outliers. This is a larger data set than is shown in the chart at the top of this section, which corresponds to Figure 7 of G. Girard’s paper.<sub> </sub></ref> |
|||
What ''is'' known specifically about the IPK is that it exhibits a short-term instability of about 30 µg over a period of about a month in its after-cleaned mass.<ref>Report to the CGPM, 14th meeting of the Consultative Committee for Units (CCU), April 2001, 2. (ii); ''General Conference on Weights and Measures, 22nd Meeting, October 2003'', which stated “The kilogram is in need of a new definition because the mass of the prototype is known to vary by several parts in 10<sup>8</sup> over periods of time of the order of a month…” ([http://www.bipm.org/utils/en/zip/CGPM22.zip 3.2 MB ZIP file, here]).<sub> </sub></ref> <!-- |
|||
-->The precise reason for this short-term instability is not understood but is thought to entail surface effects: microscopic differences between the prototypes’ polished surfaces, possibly aggravated by [[hydrogen]] absorption due to [[catalysis]] of the [[volatile organic compound]]s that slowly deposit onto the prototypes as well as the [[hydrocarbon]]-based solvents used to clean them.<ref>Citations: ''Conjecture why the IPK drifts'', R. Steiner, NIST, 11 Sept. 2007; and the BBC’s ''[http://news.bbc.co.uk/1/hi/sci/tech/7084099.stm Getting the measure of a kilogram]''.<sub> </sub></ref> |
|||
Scientists are seeing far greater variability in the prototypes than previously believed. The increasing divergence in the masses of the world’s prototypes and the short-term instability in the IPK has prompted research into improved methods to obtain a smooth surface finish using diamond-turning on newly manufactured replicas and has intensified the search for a new definition of the kilogram. See ''[[#Proposed future definitions|Proposed future definitions]]'', below.<ref>General section citations: ''Recalibration of the U.S. National Prototype Kilogram'', R. S. Davis, Journal of Research of the National Bureau of Standards, '''90''', No. 4, {{nowrap|July–August}} 1985 ([http://nvl.nist.gov/pub/nistpubs/jres/090/4/V90-4.pdf 5.5 MB PDF, here]); and ''The Kilogram and Measurements of Mass and Force'', Z. J. Jabbour ''et al.'', J. Res. Natl. Inst. Stand. Technol. '''106''', 2001, {{nowrap|25–46}} ([http://nvl.nist.gov/pub/nistpubs/jres/106/1/j61jab.pdf 3.5 MB PDF, here])<sub> </sub></ref> |
|||
==Importance of the kilogram== |
|||
[[Image:Lasertests.jpg|thumb|right|264px| The magnitude of many of the units comprising the SI system of measurement, including most of those used in the measurement of electricity and light, are dependent—to one degree or another—upon the precise mass of a {{age|1879|1|1}}-year-old, golf ball-size cylinder of metal stored in a vault in France.]] |
|||
The stability of the [[#Glossary|IPK]] is crucial because the kilogram underpins much of the SI system of measurement as it is currently defined and structured. For instance, the [[newton]] is defined as the force necessary to accelerate one kilogram at one [[Metre per second squared|meter per second squared]]. If the mass of the IPK were to change slightly, so too must the newton by a proportional degree. In turn, the [[Pascal (unit)|pascal]], the SI unit of [[pressure]], is defined in terms of the newton. This chain of dependency follows to many other SI units of measure. For instance, the [[joule]], the SI unit of [[energy]], is defined as that expended when a force of one newton acts through one [[metre|meter]]. Next to be affected is the SI unit of [[Power (physics)|power]], the [[watt]], which is one joule per second. The [[ampere]] too is defined relative to the newton, and ultimately, the kilogram. With the [[#Glossary|magnitude]] of the primary units of electricity thus determined by the kilogram, so too follow many others; namely, the [[coulomb]], [[volt]], [[Tesla (unit)|tesla]], and [[Weber (unit)|weber]]. <!-- NOTE TO EDITORS: The ohm, siemens, farad, and henry would not be affected, since in terms of basic units, they include the ampere squared in the numerator and the kilogram in the denominator, or vice versa, and the changes in these units cancel out exactly. --> Even units used in the measure of light would be affected; the [[candela]]—following the change in the watt—would in turn affect the [[Lumen (unit)|lumen]] and [[lux]]. |
|||
Because the magnitude of many of the units comprising the SI system of measurement is ultimately defined by the mass of a {{age|1879|1|1}}-year-old, golf ball-size<!--EDITORS NOTE: The “minimum” regulation-size golf ball is Ø 1.680 inches. Golf ball manufacturers typically aim for a diameter of 1.684 ±0.004 inches, which is Ø 42.77 mm. The diameter of the IPK, if it was formed into a sphere, would be Ø 44.58 mm. --> piece of metal, the quality of the IPK must be diligently protected in order to preserve the integrity of the SI system. Yet, in spite of the best stewardship, the average mass of the worldwide ensemble of prototypes and the mass of the IPK have likely diverged another {{days elapsed times factor|1989|7|1|0.0006434|1}} µg<!-- EDITORS NOTE: This factor of 0.0006434 equates to 23.5 µg per century. --> since the third periodic verification {{age|1989|1|1}} years ago.<ref>Assuming the past trend continues, whereby the mean change in mass of the first batch of replicas relative to the IPK over one hundred years was +23.5 σ<span style="margin-left:0.3em">30 µg.</span><sub> </sub></ref> Further, the world’s national metrology labs must wait for the fourth periodic verification to confirm whether the historical trends<sub> </sub>persisted. |
|||
Fortunately, ''[[#Glossary|definitions]]'' of the SI units are quite different from their ''[[#Glossary|practical realizations]].'' For instance, the [[metre|meter]] is ''defined'' as the distance light travels in a vacuum during a time interval of {{frac|299,792,458}} of a second. However, the meter’s ''practical realization'' typically takes the form of a helium-neon laser, and the meter’s length is ''[[#Glossary|delineated]]''—not defined—as {{delimitnum|1579800.298728|||}} wavelengths of light from this laser. Now suppose that the official measurement of the second was found to have drifted by a few parts per billion (it is actually exquisitely stable). There would be no automatic effect on the meter because the second—and thus the meter’s length—is [[#Glossary|abstracted]] via the laser comprising the meter’s practical realization. Scientists performing meter calibrations would simply continue to measure out the same number of laser wavelengths until an agreement was reached to do otherwise. The same is true with regard to the real-world dependency on the kilogram: if the mass of the IPK was found to have changed slightly, there would be no automatic effect upon the other units of measure because their practical realizations provide an insulating layer of abstraction. Any discrepancy would eventually have to be reconciled though because the virtue of the SI system is its precise mathematical and logical harmony amongst its units. If the IPK’s value were definitively proven to have changed, one solution would be to simply redefine the kilogram as being equal to the mass of the IPK plus an offset value, similarly to what is currently done with its replicas; e.g., “the kilogram is equal to the mass of the IPK + 42 [[Parts-per notation|ppb]]” (equivalent to 42 µg). |
|||
The long-term solution to this problem, however, is to liberate the SI system’s dependency on the IPK by developing a practical realization of the kilogram that can be reproduced in different laboratories by following a written specification. The units of measure in such a practical realization would have their magnitudes precisely defined and expressed in terms of fundamental physical constants. While major portions of the SI system would still be based on the kilogram, the kilogram would in turn be based on invariant, universal constants of nature. While this is a worthwhile objective and much work towards that end is ongoing, no alternative has yet achieved the uncertainty of a couple parts in 10<sup>8</sup> (~20 µg) required to improve upon the IPK. However, as of April 2007, the U.S.’s [[National Institute of Standards and Technology]] (NIST) had an implementation of the [[watt balance]] that was approaching this goal, with a demonstrated uncertainty of 36 µg.<ref name="IEEESteiner">''Uncertainty Improvements of the NIST Electronic Kilogram'', RL Steiner ''et al.'', Instrumentation and Measurement, IEEE Transactions on, '''56''' Issue 2, April 2007, {{nowrap|592–596}}<sub> </sub></ref> See ''[[#Watt balance|Watt balance]]'', below. |
|||
== Proposed future definitions == |
|||
<!--NOTE TO EDITORS: This section is externally linked to from other places in the article. Please do not delete or rename without fixing the referencing links.--> |
|||
:''In the following section, wherever numeric equalities are shown in ‘concise form’—such as'' {{val|1.85487|(14)|e=43}}''—the two digits between the parentheses denotes the [[uncertainty]] at ''1σ'' [[standard deviation]] ''(68%'' confidence level) in the two least significant digits of the [[significand]].'' |
|||
The kilogram is the only SI unit that is still defined in relation to an artifact. Note that the [[Metre|meter]] was also once defined as an artifact (a single platinum-iridium bar with two marks on it). However, it was eventually redefined in terms of invariant, fundamental constants of nature that are delineated via ''practical realizations'' (apparatus) that can be reproduced in different laboratories by following a written specification. Today, physicists are investigating various approaches to do the same with the kilogram. Some of the approaches are fundamentally very different from each other. Some are based on equipment and procedures that enable the reproducible production of new, kilogram-mass prototypes on demand (albeit with extraordinary effort) using measurement techniques and material properties that are ultimately based on, or traceable to, fundamental constants. Others are devices that measure either the acceleration or weight of hand-tuned, kilogram test masses and which express their [[#Glossary|magnitudes]] in electrical terms via special components that permit traceability to fundamental constants. Measuring the weight of test masses requires the precise measurement of the strength of gravity in laboratories. All approaches would precisely fix one or more constants of nature at a defined value. These different approaches are as follows: |
|||
====Atom-counting approaches==== |
|||
=====Carbon-12===== |
|||
Though not offering a practical realization, this definition would precisely define the magnitude of the kilogram in terms of a certain number of [[carbon-12|carbon‑12]] atoms. Carbon‑12 (<sup>12</sup>C) is an [[isotope]] of carbon. The [[mole (unit)|mole]] is currently defined as “the quantity of entities (elementary particles like atoms or molecules) equal to the number of atoms in 12 grams of carbon‑12.” Thus, the current definition of the mole requires that {{frac|1000|12}}=83⅓ moles of <sup>12</sup>C has a mass of precisely one kilogram. The number of atoms in a mole, a quantity known as the [[Avogadro constant]], is an experimentally determined value that is currently measured as being {{val|6.02214179|(30)|e=23}} atoms (2006 [[Committee on Data for Science and Technology|CODATA]] value). This new definition of the kilogram proposes to fix the Avogadro constant at precisely {{val|6.02214179||e=23}} and the kilogram would be defined as “the mass equal to that of {{nowrap|{{frac|1000|12}} · {{val|6.02214179||e=23}} }}atoms of <sup>12</sup>C.” |
|||
Currently, the uncertainty in the Avogadro constant is determined by the uncertainty in the measured mass of <sup>12</sup>C atoms (a relative standard uncertainty of 50 [[Parts-per notation|parts per billion]] at this time). By fixing the Avogadro constant, the practical effect of this proposal would be that the precise magnitude of the kilogram would be subject to future refinement as improved measurements of the mass of <sup>12</sup>C atoms become available; electronic realizations of the kilogram would be recalibrated as required. Conversely, an ''electronic'' definition of the kilogram would continue to allow 83⅓ moles ({{frac|1000|12}} moles) of <sup>12</sup>C to have a mass of precisely one kilogram but the Avogadro constant would continue to be subject to future refinement. |
|||
A variation on a <sup>12</sup>C-based definition proposes to define the Avogadro constant as being precisely 84,446,886<sup>3</sup> |
|||
(≈6.022<span style="margin-left:0.25em">140<span style="margin-left:0.25em">98</span> × 10<sup>23</sup>) <!-- DO NOT use {{val}} as it does not properly display this particular value-->atoms. An imaginary realization of a 12-gram mass prototype would be a cube of <sup>12</sup>C atoms measuring precisely 84,446,886 atoms across on a side. With this proposal, the kilogram would be defined as “the mass equal to 84,446,886<sup>3</sup> × 83⅓ atoms of <sup>12</sup>C." The value 84,446,886 was chosen because it has a special property; its cube (the proposed new value for the Avogadro constant) is evenly divisible by twelve. Thus with this definition of the kilogram, there would be an integer number of atoms in one gram of <sup>12</sup>C: 50,184,508,190,229,061,679,538 atoms.<!-- |
|||
--><ref>Georgia Tech, ''[http://www.gatech.edu/news-room/release.php?id=1513 “ A Better Definition for the Kilogram?”]'' 21 September 2007 (press release): Note that the uncertainty in the Avogadro constant narrowed since this proposal was first submitted to ''[[American Scientist]]'' for publication. The 2006 CODATA value for the Avogadro constant has a relative standard uncertainty of 50 parts per billion and the only cube root values within this uncertainty must fall within the range of 84,446,889.8 ±1.4; that is, there are only three integer cube roots (…89, …90, and …91) in this range and the value 84,446,886 falls outside of it. Unfortunately, none of the three integer values within the range possess the property of their cubes being divisible by twelve; one gram of <sup>12</sup>C could not comprise an integer number of atoms. If the value 84,446,886 was adopted to define the kilogram, many other constants of nature and electrical units would have to be revised an average of about 0.13 part per million. The straightforward adjustment to this approach advanced by the group would instead define the kilogram as “the mass equal to 84,446,890<sup>3</sup> × 83⅓ atoms of carbon‑12.” This proposed value for the Avogadro constant (≈6.022<span style="margin-left:0.25em">141<span style="margin-left:0.2em">84</span> × 10<sup>23</sup>)<!-- DO NOT USE {{val}} for this value as it has a bug that improperly displays it. --> falls neatly within the 2006 CODATA value of {{val|6.02214179|(30)|e=23}}) and the proposed definition of the kilogram produces an integer number of atoms in 12 grams of carbon‑12 (602,214,183,858,071,454,769,000 atoms), but not for 1 gram or 1 kilogram.</ref> |
|||
=====Avogadro project===== |
|||
[[Image:Silicon sphere for Avogadro project.jpg|thumb|right|Shown above, one of the master opticians at the [http://www.csiro.au/acpo/ Australian Centre for Precision Optics] (ACPO) is holding a 1 kg, single-crystal silicon sphere for the Avogadro project. These spheres are among the roundest man-made objects in the world. If the best of these spheres were scaled to the size of Earth, its high point—a continent-size area—would gently rise to a maximum elevation of only 2.4 m above “sea level.”<ref>The sphere shown in the photograph has an out-of-roundness value (peak to valley on the radius) of 50 nm. According to ACPO, they improved on that with an out-of-roundness of 35 nm. On the 93.6 mm diameter sphere, an out-of-roundness of 35 nm (undulations of ±17.5 nm) is a fractional roundness (∆r/r) = {{val|3.7|e=-7}}. Scaled to the size of Earth, this is equivalent to a maximum deviation from sea level of only 2.4 m. The roundness of that ACPO sphere is exceeded only by two of the four [[Fused quartz|fused-quartz]] gyroscope rotors flown on [[Gravity Probe B|Gravity Probe B]], which were manufactured in the late 1990s and given their final figure at the [http://hepl.stanford.edu/ W.W. Hansen Experimental Physics Lab] at [[Stanford University]]. Particularly, “Gyro 4” is recorded in the [[Guinness world records|Guinness]] database of world records (their database, not in their book) as ''the'' world’s roundest man-made object. According to a published report ([http://aa.stanford.edu/aeroastro/posters2007/Polhode_Motion.pdf 221 kB PDF, here]<span style="margin-left:0.2em">)</span> and the GP‑B public affairs coordinator at Stanford University, of the four gyroscopes onboard the probe, Gyro 4 has a maximum surface undulation from a perfect sphere of 3.4 ±0.4 nm on the 38.1 mm diameter sphere, which is a ∆r/r = {{val|1.8|e=-7}}. Scaled to the size of Earth, this is equivalent to an undulation the size of North America rising slowly up out of the sea (in molecular-layer terraces 11.9 cm high), reaching a maximum elevation of 1.14 ±0.13 m in Nebraska, and then gradually sloping back down to sea level on the other side of the continent. The only known objects in the universe that are rounder than this are [[neutron star]]s.</ref>]]<!-- |
|||
-->Another Avogadro constant-based approach, known as the ''Avogadro project'', would define and delineate the kilogram as a softball-size (93.6 mm diameter) sphere of [[silicon]] atoms. Silicon was chosen because a commercial infrastructure with mature processes for creating defect-free, ultra-pure monocrystalline silicon already exists to service the [[semiconductor]] industry. To make a practical realization of the kilogram, a silicon [[Boule (crystal)|boule]] (a rod-like, single-crystal ingot) would be produced. Its [[isotope|isotopic]] composition would be measured with a [[Mass spectrometry|mass spectrometer]] to determine its average atomic mass. The rod would be cut, ground, and polished into spheres. The size of a select sphere would be measured using optical [[interferometry]] to an uncertainty of about 0.3 nm on the radius—roughly a single atomic layer. The precise lattice spacing between the atoms in its crystal structure (≈192 pm) would be measured using a scanning X-ray interferometer. This permits its atomic spacing to be determined with an uncertainty of only three parts per billion. With the size of the sphere, its average atomic mass, and its atomic spacing known, the required sphere diameter can be calculated with sufficient precision and uncertainty to enable it to be finish-polished to a target mass of one kilogram. |
|||
Experiments are being performed on the Avogadro Project’s silicon spheres to determine whether their masses are most stable when stored in a vacuum, a partial vacuum, or ambient pressure. However, no technical means currently exist to prove a long-term stability any better than that of the IPK’s because the most sensitive and accurate measurements of mass are made with [[Mass_versus_weight#Types of scales and what they measure|dual-pan]] [[Weighing_scale#Balance|balances]] like the BIPM’s FB‑2 flexure-strip balance (see ''[[#External links|External links]]'', below). Balances can only compare the mass of a silicon sphere to that of a reference mass. Given the latest understanding of the lack of long-term mass stability with the IPK and its replicas, there is no known, perfectly stable mass artifact to compare against. [[Mass_versus_weight#Types of scales and what they measure|Single-pan]] [[Weighing scale|scale]]s capable of measuring weight relative to an invariant of nature with a long-term uncertainty of only {{nowrap|10–20}} [[Parts-per notation|parts per billion]] do not yet exist. Another issue to be overcome is that silicon oxidizes and forms a thin layer (equivalent to {{nowrap|5–20}} silicon atoms) of [[silicon dioxide]] (common glass) and silicon monoxide. This layer slightly increases the mass of the sphere, an effect which must be accounted for when polishing the sphere to its finish dimension. Oxidation is not an issue with platinum and iridium, both of which are [[noble metal]]s that are roughly as [[Galvanic series|cathodic]] as oxygen and therefore don’t oxidize unless coaxed to do so in the laboratory. The presence of the thin oxide layer on a silicon-sphere mass prototype places additional restrictions on the procedures that might be suitable to clean it to avoid changing the layer’s thickness or oxide [[stoichiometry]]. |
|||
All silicon-based approaches would fix the Avogadro constant but vary in the details of the definition of the kilogram. One approach would use silicon with all three of its natural isotopes present. About 7.77% of silicon comprises the two heavier isotopes: <sup>29</sup>Si and <sup>30</sup>Si. As described in ''[[#Carbon-12|Carbon‑12]]'' above, this method would ''define'' the magnitude of the kilogram in terms of a certain number of <sup>12</sup>C atoms by fixing the Avogadro constant; the silicon sphere would be the ''practical realization.'' This approach could accurately delineate the magnitude of the kilogram because the mass of the three silicon isotopes relative to <sup>12</sup>C are known with great precision. An alternative method for creating a silicon sphere-based kilogram proposes to use [[Isotope separation|isotopic separation]] techniques to enrich the silicon until it is nearly pure <sup>28</sup>Si, which has an atomic mass of {{val|27.9769271|(7)|u=g/mol}}. With this approach, the Avogadro constant would not only be fixed, but so too would the atomic mass of <sup>28</sup>Si. As such, the definition of the kilogram would be decoupled from <sup>12</sup>C and the kilogram would instead be defined as {{frac|1000|{{val|27.9769271}}}} '''·''' {{val|6.02214179|e=23}} atoms of <sup>28</sup>Si (≈{{val|35.743739705}} fixed moles of <sup>28</sup>Si atoms). Physicists could elect to define the kilogram in terms of <sup>28</sup>Si even when kilogram prototypes are made of natural silicon (all three isotopes present). Even with a kilogram definition based on <sup>28</sup>Si, a silicon-sphere prototype made of nearly pure <sup>28</sup>Si would necessarily deviate slightly from the defined number of moles of silicon in order to compensate for various chemical and isotopic impurities as well as the effect of surface oxides.<ref>Citations: NPL: ''[http://www.npl.co.uk/server.php?show=ConWebDoc.2050 Avogadro Project]''; Australian National Measurement Institute: ''[http://www.measurement.gov.au/index.cfm?event=object.showContent&objectID=7CBA5E62-BCD6-81AC-15017BE81748EF4B Redefining the kilogram through the Avogadro constant]''; and Australian Centre for Precision Optics: ''[http://www.acpo.csiro.au/avogadro.htm The Avogadro Project]''</ref> |
|||
=====Ion accumulation===== |
|||
Another Avogadro-based approach, [[ion]] accumulation, would define and delineate the kilogram by precisely creating new metal prototypes on demand. It would do so by accumulating [[gold]] or [[bismuth]] [[ion]]s (atoms stripped of an electron) and counting them by measuring the electrical current required to neutralize the ions. Gold (<sup>197</sup>Au) and bismuth (<sup>209</sup>Bi) were chosen because they can be safely handled and have the two highest [[atomic mass]]es among the [[mononuclidic elements]] that can be treated as if it is virtually non-radioactive (bismuth) or is perfectly stable (gold). See also ''[[Table of nuclides (combined)|Table of nuclides]]''.<ref>In 2003, the same year the first gold-deposition experiments were conducted, physicists found that the only naturally occurring isotope of bismuth, <sup>209</sup>Bi, is actually very slightly [[Radioactive decay|radioactive]], with the longest known radioactive [[half-life]] of any naturally occurring element that decays via [[Alpha particle|alpha radiation]]—a half-life of {{val|19|2|e=18|u=years}}. As this is 1.4 billion times the age of the universe, <sup>209</sup>Bi is considered a stable isotope for most practical applications (those unrelated to such disciplines as [[nucleocosmochronology]] and [[geochronology]]). In other terms, {{nowrap|{{val|99.999999983}}%}} of the bismuth that existed on Earth 4.567 billion years ago still exists today. Only two mononuclidic elements are heavier than bismuth and only one approaches its stability: [[thorium]]. Long considered a possible replacement for uranium in nuclear reactors, thorium can cause cancer when inhaled because it is over 1.2 billion times more radioactive than bismuth. It also has such a strong tendency to oxidize that its powders are [[Pyrophoricity|pyrophoric]]. These characteristics make thorium unsuitable in ion-deposition experiments. See also ''[[Isotopes of bismuth]]'', ''[[Isotopes of gold]]'' and ''[[Isotopes of thorium]]''.</ref> |
|||
With a gold-based definition of the kilogram for instance, the atomic mass of gold would be fixed as precisely {{val|196.966569|u=g/mol}}, from the current value of {{val|196.966569|(4)|u=g/mol}}. As with a definition based upon carbon‑12, the Avogadro constant would also be fixed. The kilogram would then be defined as “the mass equal to that of precisely {{frac|1000|{{val|196.966569}}}} '''·''' {{val|6.02214179|e=23}} atoms of gold” (precisely 3,057,443,616,231,138,188,734,962 atoms of gold or about about {{val|5.077003702}} fixed moles). |
|||
Ion-accumulation techniques, while a relatively new field of study, have advanced rapidly. In 2003, experiments with gold at a current of only 10 µA demonstrated a relative uncertainty of 1.5%.<ref name="PTB">The German national metrology institute, known as the Physikalisch-Technische Bundesanstalt (PTB): [http://www.ptb.de/en/org/1/12/124/ionenex.htm Working group 1.24, Ion Accumulation]</ref> Yet, follow-on experiments using bismuth ions and a current of 30 mA were expected to accumulate a mass of 30 g in six days and to have a relative uncertainty of better than 1 part in 10<sup>6</sup>.<ref>[http://www.bipm.org/utils/en/zip/CGPM22.zip ''General Conference on Weights and Measures, 22nd Meeting, October 2003''] (3.2 MB ZIP file).</ref> |
|||
Among the many technical challenges of the ion-deposition apparatus is in obtaining a sufficiently high ion current (mass deposition rate) while simultaneously decelerating the ions so they can all deposit onto the target electrode imbedded in a balance pan. Experiments with gold showed the ions had to be decelerated to very low energies to avoid [[sputtering]] effects—an effect wherein ions that have already been counted either ricochet off or are disloged from a target electrode. The deposited mass fraction in the 2003 experiments approached very closely to 100% only at ion energies of less than around 1 [[Electronvolt|eV]] (<1 km/s for gold).<ref name="PTB"/> |
|||
If the kilogram is one day defined as a precise quantity of gold or bismuth atoms deposited with an electric current, not only would the Avogadro constant and the atomic mass of gold or bismuth be precisely fixed, but so too would the value of the [[ampere]]. This would be brought about by precisely fixing the value of the [[elementary charge]] (''e''<span style="margin-left:0.1em">)</span>, likely to {{val|1.602176487|e=-19}} [[coulomb]] (from the present 2006 CODATA value of {{val|1.602176487|(40)|e=-19}}). Doing so would effectively define the ampere as a flow of {{frac|{{val|1.602176487|e=-19}}}} (6,241,509,647,120,417,390) electrons per second past a fixed point in an electric circuit. The SI unit of mass would thus be fully defined by having precisely fixed the values of the Avogadro constant and elementary charge, and by exploiting the fact that the atomic masses of bismuth and gold atoms are invariant, universal constants of nature. |
|||
One challenge in maturing an ion-accumulation-based kilogram into a practical realization is the development of a deposition chamber/balance system that enables the convenient calibration of a reasonable quantity of [[#Glossary|transfer standards]] relative to any single internal ion-deposited prototype. The mass prototypes produced by ion deposition techniques are nothing like the freestanding platinum-iridium prototypes currently in use; they are deposited onto—and become part of—an electrode imbedded into one pan of a special balance that is part of the device. Further, the ion-deposited material doesn’t have a hard, highly polished surface that can be vigorously cleaned like those of current prototypes. Gold, while dense and a [[noble metal]] (resistant to oxidation and the formation of other compounds), is extremely soft so an internal gold prototype would have to be kept well isolated and scrupulously clean to avoid contamination and the potential of wear from having to remove the contamination. Bismuth, which is an inexpensive metal used in low-temperature solders, slowly oxidizes when exposed to room-temperature air and forms other chemical compounds and so would not produce a stable reference mass unless it was continually maintained in a vacuum or inert atmosphere. |
|||
====Electronic approaches==== |
|||
=====Watt balance===== |
|||
[[Image:Watt balance, large view.jpg|thumb|right|275px|The [[National Institute of Standards and Technology|NIST’s]] watt balance is a project of the U.S. Government to develop an “electronic kilogram.” The vacuum chamber dome, which lowers over the entire apparatus, is visible at top.]]The [[watt balance]] is essentially a [[Mass_versus_weight#Types of scales and what they measure|single-pan]] [[weighing scale]] that measures the [[electric power]] necessary to oppose the weight of a kilogram test mass as it is accelerated by gravity. It is a variation of an [[ampere balance]] in that it employs an extra calibration step that nulls the effect of geometry. The [[electric potential]] in the watt balance is delineated by a [[Josephson effect|Josephson voltage standard]], which allows voltage to be linked to an invariant constant of nature with extremely high precision and stability. Its circuit [[Electrical resistance|resistance]] is calibrated against a [[Hall effect|quantum Hall resistance standard]]. The watt balance requires exquisitely precise measurement of gravity in a laboratory (see “FG‑5 absolute gravimeter” in ''[[#External links |External links]]'', below). For instance, the gravity gradient of 3.1 [[Gal (unit)|µGal]]/cm (≈3 µg/cm) is accounted for when the elevation of the center of the [[gravimeter]] differs from that of the nearby test mass. As of April 2007, the [[National Institute of Standards and Technology|NIST’s]] implementation of the watt balance was demonstrating a combined relative standard uncertainty (CRSU) at 68% probability of 36 µg and a short-term resolution of {{nowrap|10–15}} µg.<ref name="IEEESteiner"/> The UK’s [[National Physical Laboratory, UK|National Physical Laboratory’s]] watt balance was demonstrating a CRSU of 70.3 µg as of 2007.<ref>"An initial measurement of Planck's constant using the NPL Mark II watt balance", I.A. Robinson ''et al.'', ''Metrologia'' '''44''' (2007), {{nowrap|427–440;}}<br> NPL: ''[http://www.npl.co.uk/server.php?show=ConWebDoc.2029 NPL Watt Balance]''</ref> |
|||
Ultimately, the watt balance would define the kilogram in terms of the [[Planck constant]], which is a measure that relates the energy of photons to their frequency. The Planck constant would be fixed, where ''h'' = {{val|6.62606896|e=-34|u=[[Joule-second|J·s]]}} (from the 2006 CODATA value of {{val|6.62606896|(33)|e=-34|u=J·s}}) and the kilogram would be defined as “the mass of a body at rest whose equivalent energy equals the energy of photons whose frequencies sum to {{val|1.356392733|e=50|u=Hz}}.”<ref> |
|||
{{cite journal |
|||
|author=Joshua P Schwarz ''et al.'' |
|||
|url=http://nvl.nist.gov/pub/nistpubs/jres/106/4/j64schw.pdf |
|||
|title=Hysteresis and Related Error Mechanisms in the NIST Watt Balance Experiment |
|||
|format=888 KB PDF |
|||
|journal=Journal of Research of the National Bureau of Standards and Technology |
|||
|volume=106 |
|||
|issue=4 |
|||
|month={{nowrap|July–August}} |
|||
|year=2001 |
|||
}}<br> |
|||
{{cite journal |
|||
|title=On the redefinition of the kilogram |
|||
|author=B.N. Taylor ''et al.'' |
|||
|journal=Metrologia |
|||
|volume=36 |
|||
|year=1999 |
|||
|pages=63–64 |
|||
}}<br> |
|||
{{cite web |
{{cite web |
||
|url = http://www.bipm.org/utils/en/pdf/si_brochure_draft_ch2.pdf |
|||
|url=http://physics.nist.gov/cgi-bin/cuu/Convert?exp=0&num=1&From=kg&To=hz&Action=Convert+value+and+show+factor |
|||
|title = Draft Chapter 2 for SI Brochure, following redefinitions of the base units |
|||
|publisher=NIST |
|||
|author = Ian Mills |
|||
|title=Fundamental physical constants: “Energy Equivalents” calculator |
|||
|publisher = CCU |
|||
}}</ref> |
|||
|date = September 29, 2010 |
|||
|access-date =January 1, 2011 |
|||
}}</ref> This resolution was accepted by the 24th conference of the CGPM<ref> |
|||
{{cite conference |
|||
|url= http://www.bipm.org/utils/en/pdf/24_CGPM_Resolution_1.pdf |
|||
|title= Resolution 1 – On the possible future revision of the International System of Units, the SI |
|||
|conference= 24th meeting of the General Conference on Weights and Measures |
|||
|location = Sèvres, France |
|||
|date = October 17–21, 2011 |
|||
|access-date =October 25, 2011 |
|||
}}</ref> in October 2011 and further discussed at the 25th conference in 2014.<ref name=":0">{{Cite web|url=http://www.bipm.org/en/CGPM/db/25/1/|title=BIPM – Resolution 1 of the 25th CGPM|website=www.bipm.org|access-date=March 27, 2017}}</ref><ref> |
|||
{{cite press release |
|||
| url = http://www.bipm.org/utils/en/pdf/Press_release_resolution_1_CGPM.pdf |
|||
| title = General Conference on Weights and Measures approves possible changes to the International System of Units, including redefinition of the kilogram. |
|||
| publisher = [[General Conference on Weights and Measures]] |
|||
| location = Sèvres, France |
|||
| date = October 23, 2011 |
|||
| access-date = October 25, 2011 |
|||
}}</ref> Although the Committee recognised that significant progress had been made, they concluded that the data did not yet appear sufficiently robust to adopt the revised definition, and that work should continue to enable the adoption at the 26th meeting, scheduled for 2018.<ref name=":0" /> Such a definition would theoretically permit any apparatus that was capable of delineating the kilogram in terms of the Planck constant to be used as long as it possessed sufficient precision, accuracy and stability. The [[Kibble balance]] is one way to do this.<ref>{{cite journal |doi=10.1088/0026-1394/53/5/A46 |title=The watt or Kibble balance: A technique for implementing the new SI definition of the unit of mass |journal=Metrologia |volume=53 |issue=5 |pages=A46–A74 |year=2016 |last1=Robinson |first1=Ian A. |last2=Schlamminger |first2=Stephan |pmid=35023879 |pmc=8752041 |bibcode=2016Metro..53A..46R |doi-access=free }}</ref> |
|||
As part of this project, a variety of very [[Alternative approaches to redefining the kilogram|different technologies and approaches]] were considered and explored over many years. Some of these approaches were based on equipment and procedures that would enable the reproducible production of new, kilogram-mass prototypes on demand (albeit with extraordinary effort) using measurement techniques and material properties that are ultimately based on, or traceable to, physical constants. Others were based on devices that measured either the acceleration or weight of hand-tuned kilogram test masses and which expressed their magnitudes in electrical terms via special components that permit traceability to physical constants. All approaches depend on converting a weight measurement to a mass and therefore require precise measurement of the strength of gravity in laboratories ([[gravimetry]]). All approaches would have precisely fixed one or more constants of nature at a defined value.{{Citation needed|date=July 2023}} |
|||
[[Image:Michelson Interferometer Laser Interference Fringes-Red.jpg|thumb|left|239px|Gravity is measured with exceptional precision with the help of a laser interferometer. The laser’s pattern of [[Interference|interference fringes]]—the dark and light bands above—blooms at an ever faster rate as a free-falling [[Retroreflector|corner reflector]] drops inside an absolute [[gravimeter]]. The pattern’s frequency sweep is timed by an atomic clock.]] The virtue of electronic realizations like the watt balance is that the definition and [[#Glossary|dissemination]] of the kilogram would no longer be dependent upon the stability of kilogram prototypes, which must be very carefully handled and stored. It would free physicists from the need to rely on assumptions about the stability of those prototypes, including those that would be manufactured under atom-counting schemes. Instead, hand-tuned, close-approximation mass standards would simply be weighed and documented as being equal to one kilogram plus an offset value. With scales, the kilogram would not only be ''defined'' in electrical terms, it would also be ''delineated'' in electrical terms. Mass artifacts calibrated in a watt balance would effectively become ''[[#Glossary|transfer standards]]''. Further, one additional term in all scale-based realizations—acceleration due to gravity—is currently measured using dropping-mass absolute [[gravimeter]]s that contain an iodine-stabilized [[helium-neon laser]] [[Interferometry|interferometer]]. The [[Interference|fringe-signal]], [[Chirp|frequency-sweep]] output from the interferometer is measured with a rubidium [[atomic clock]]. Thus, the ‘gravity’ term in the delineation of an all-electronic kilogram would also be measured relative to invariants of nature. |
|||
Scales like the watt balance also permit more flexibility in choosing materials with especially desirable properties for mass standards. For instance, {{nowrap|Pt-10Ir}} could continue to be used so that the specific gravity of newly produced mass standards would be the same as existing national primary and check standards (≈21.55 g/ml). This would reduce the relative uncertainty when making [[Mass versus weight#Buoyancy and “conventional mass”|mass comparisons in air]]. Alternately, entirely different materials and constructions could be explored with the objective of producing mass standards with greater stability. For instance, [[osmium]]-iridium alloys could be investigated if platinum’s propensity to absorb hydrogen (due to catalysis of VOCs and hydrocarbon-based cleaning solvents) and atmospheric [[Mercury (element)|mercury]] proved to be sources of instability. Also, vapor-deposited, protective ceramic coatings like [[Titanium nitride|nitrides]] could be investigated for their suitability to isolate these new alloys. |
|||
The challenge with watt balances is not only in reducing their uncertainty, but also in making them truly ''practical'' realizations of the kilogram. Nearly every aspect of watt balances and their support equipment requires such extraordinarily precise and accurate, state-of-the-art technology that—unlike a device like an atomic clock—few countries would currently choose to fund their operation. For instance, the NIST’s watt balance used four resistance standards, each of which was rotated through the watt balance every two to six weeks after being calibrated in a different part of its [[Gaithersburg, Maryland]] [[NIST#Facilities|facility]]. It was found that simply moving the resistance standards down the hall to the watt balance after calibration altered their values 10 [[Parts-per notation|ppb]] (equivalent to 10 µg) or more. Present-day technology is insufficient to permit stable operation of watt balances between even biannual calibrations. If the kilogram is defined in terms of the Planck constant, it is likely there will only be a few—at most—watt balances initially operating in the world. |
|||
=====Ampere-based force===== |
|||
[[Image:Meissner effect zoom.jpg|thumb|right|A magnet floating above a superconductor bathed in liquid nitrogen demonstrates [[Superdiamagnetism|perfect diamagnetic]] levitation via the [[Meissner effect]]. Experiments with an ampere-based definition of the kilogram flipped this arrangement upside-down: an electric field accelerated a superconducting test mass supported by fixed magnets.]] |
|||
This approach would define the kilogram as “the mass which would be accelerated at precisely {{val|2|e=-7|u=m/s²}} when subjected to the per-meter force between two straight parallel conductors of infinite length, of negligible circular cross section, placed one meter apart in vacuum, through which flow a constant current of {{frac|({{val|1.602176487|e=-19}})}}≈6,241,509,647,120,417,390 elementary charges per second.” |
|||
Effectively, this would define the kilogram as a derivative of the [[ampere]], rather than present relationship, which defines the ampere as a derivative of the kilogram. This redefinition of the kilogram would result from changing the [[elementary charge]] (''e'') to be fixed at precisely {{val|1.602176487|e=-19}} [[coulomb]] rather than the current 2006 CODATA value of {{val|1.602176487|(40)|e=-19}}, which effectively defines the coulomb as being the sum of 6,241,509,647,120,417,390 elementary charges. It would necessarily follow that the ampere then becomes an electrical current of this same quantity of elementary charges per second. |
|||
The virtue of a practical realization based upon this definition is that unlike the watt balance and other scale-based methods, all of which require the careful characterization of gravity in the laboratory, this method delineates the magnitude of the kilogram directly in the very terms that define the nature of mass: acceleration due to an applied force. Unfortunately, it is extremely difficult to develop a practical realization based upon accelerating masses. Experiments over a period of years in Japan with a [[Superconductivity|superconducting]], 30 g mass supported by [[Diamagnetism|diamagnetic]] levitation never achieved an uncertainty better than ten parts per million. [[Hysteresis#Magnetic hysteresis|Magnetic hysteresis]] was one of the limiting issues. Other groups are continuing this line of research using different techniques to levitate the mass.<ref>{{cite press release |publisher=NIST |url=http://www.nist.gov/public_affairs/newsfromnist_beyond_the_kilogram.htm |title=Beyond the kilogram: redefining the International System of Units}}<br>R. Steiner, ''A Watt Balance On Its Side'', NIST, 24 September 2007.</ref> |
|||
== SI multiples == |
== SI multiples == |
||
<!--NOTE TO EDITORS: This section is linked from [[Caffeine]] as well as many other Wikipedia articles. Please do not delete or rename without fixing them. |
{{main|Orders of magnitude (mass)}}<!--NOTE TO EDITORS: This section is linked from [[Caffeine]] as well as many other Wikipedia articles. Please do not delete or rename without fixing them.--> |
||
{{Redirect|Milligram|the band|Milligram (band)|the horse|Milligram (horse)}} |
|||
Because an SI unit may not have multiple prefixes (see [[SI prefix]]), prefixes are added to ''[[gram]]'', rather than the base unit ''kilogram'', which already has a prefix as part of its name.<ref>BIPM: SI Brochure: Section 3.2, ''[http://www.bipm.org/en/publications/si-brochure/section3-2.html The kilogram]'' {{Webarchive|url=https://web.archive.org/web/20160329162832/http://www.bipm.org/en/publications/si-brochure/section3-2.html |date=March 29, 2016 }}</ref> For instance, one-millionth of a kilogram is 1{{nbsp}}mg (one milligram), not 1{{nbsp}}μkg (one microkilogram). |
|||
--> |
|||
Because [[SI prefix]]es may not be concatenated (serially linked) within the name or symbol for a unit of measure, SI prefixes are used with the ''[[gram]]'', not the kilogram, which already has a prefix as part of its name.<ref>BIPM: SI Brochure: Section 3.2, ''[http://www.bipm.org/en/si/si_brochure/chapter3/3-2.html The kilogram]<sub> </sub></ref> For instance, one-millionth of a kilogram is 1 mg (one milligram), not 1 µkg (one microkilogram). |
|||
<div style="float:center; margin-left: 1em;"> |
<div style="float:center; margin-left: 1em;"> |
||
{{SI multiples |
{{SI multiples |
||
|unit=gram |
| unit = gram |
||
|symbol=g |
| symbol = g |
||
| p= | n= | mc= | m= | k= | M= | G= | T= |
|||
|note=Common prefixes are in bold face.<ref>Criterion: A combined total of at least 250,000 Google hits on both the U.S. spelling (‑gram) and the U.K./International spelling (‑gramme).<sub> </sub></ref> |
|||
| xM = megagram''' ([[tonne]])''' |
|||
|p= | n= | µ= | m= | k= |
|||
| note = Common prefixed units are in bold face.<ref group="Note">Criterion: A combined total of at least five occurrences on the [[British National Corpus]] and the [[Corpus of Contemporary American English]], including both the singular and the plural for both the -''gram'' and the -''gramme'' spelling.</ref> |
|||
|xM = megagram ([[tonne]]) |
|||
|xmc = microgram (mcg) |
|||
}} |
}} |
||
</div> |
</div> |
||
<br><!-- |
|||
== Practical issues with SI weight names == |
|||
EDITORS NOTE REGARDING BULLET-POINT TEXT SIZE: The below bullet notes have alternately been tried in both normal-size text, then small text, then normal, etc. While it may be tempting to reduce the below section (as they are near-parenthetical, footnote-type notes), small text has proven too difficult to read on many computer systems. Accordingly, please leave the below text in normal size. --> |
|||
<!--EDITORS NOTE REGARDING BULLET-POINT TEXT SIZE: The below bullet notes have alternately been tried in both normal-size text, then small text, then normal, etc. While it may be tempting to reduce the below section (as they are near-parenthetical, footnote-type notes), small text has proven too difficult to read on many computer systems. Accordingly, please leave the below text in normal size. --> |
|||
* When the Greek lowercase “µ” <!--NOTE TO EDITORS: Technically, the “µ” symbol used throughout this article is the special Unicode µ symbol ( µ ), not the μ symbol. This reader is spared this detail of HTML coding.-->(mu) in the symbol of microgram is typographically unavailable, it is occasionally—although not properly—replaced by Latin lowercase “u”. |
|||
* Serious medication errors have been made by confusing milligrams and micrograms when micrograms has been abbreviated.<ref name="SPCG">{{cite web |url=http://www.palliativecareguidelines.scot.nhs.uk/guidelines/about-the-guidelines/pharmacological-considerations/prescribing-information-for-liquid-medicines.aspx |title=Prescribing Information for Liquid Medicines |website=Scottish Palliative Care Guidelines |access-date=June 15, 2015 |archive-url=https://web.archive.org/web/20180710152658/http://www.palliativecareguidelines.scot.nhs.uk/guidelines/about-the-guidelines/pharmacological-considerations/prescribing-information-for-liquid-medicines.aspx |archive-date=July 10, 2018 |url-status=dead }}</ref> The abbreviation "mcg" rather than the SI symbol "μg" is formally mandated for medical practitioners in the US by the [[Joint Commission on Accreditation of Healthcare Organizations]] (JCAHO).<ref>{{cite web |title=New Joint Commission "Do Not Use" List: Abbreviations, Acronyms, and Symbols |url=http://www.aapmr.org/practice/guidelines/Pages/New-Joint-Commission-symbols.aspx |publisher=American Academy of Physical Medicine and Rehabilitation |access-date=19 February 2024 |archive-url=https://web.archive.org/web/20150915012112/http://www.aapmr.org/practice/guidelines/Pages/New-Joint-Commission-symbols.aspx |archive-date=15 September 2015}}</ref> In the United Kingdom, the [[National Institute for Health and Care Excellence]] and Scottish Palliative Care Guidelines state that "micrograms" and "nanograms" must both be written in full, and never abbreviated as "mcg" or "μg".<ref name="SPCG" /><ref>{{cite web |title=Prescription writing |url=https://bnf.nice.org.uk/medicines-guidance/prescription-writing/ |publisher=[[National Institute for Health and Care Excellence]] |access-date=19 February 2024}}</ref> |
|||
* The microgram is often abbreviated “mcg”, particularly in pharmaceutical and nutritional supplement labeling, to avoid confusion since the “µ” prefix is not well recognized outside of technical disciplines.<ref>The practice of using the abbreviation “mcg” rather than the SI symbol “µg” was formally mandated for medical practitioners in 2004 by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) in their [http://www.aapmr.org/hpl/pracguide/jcahosymbols.htm “Do Not Use” List: Abbreviations, Acronyms, and Symbols] because hand-written expressions of “µg” can be confused with “mg”, resulting in a thousand-fold overdosing. The mandate was also adopted by the [http://www.ismp.org/ Institute for Safe Medication Practices.]<sub> </sub></ref> Note however, that the ''abbreviation'' “mcg”, is also the ''symbol'' for an obsolete [[Centimetre gram second system of units|CGS]] unit of measure known as the “millicentigram”, which is equal to 10 µg. |
|||
* The hectogram (100 g) (Italian: ''ettogrammo'' or ''etto'') is a very commonly used unit in the retail food trade in Italy.<ref>Tom Stobart, ''The Cook's Encyclopedia'', 1981, p. 525</ref><ref>J.J. Kinder, V.M. Savini, ''Using Italian: A Guide to Contemporary Usage'', 2004, {{isbn|0521485568}}, p. 231</ref><ref>Giacomo Devoto, Gian Carlo Oli, ''Nuovo vocabolario illustrato della lingua italiana'', 1987, ''s.v.'' 'ètto': "frequentissima nell'uso comune: ''un e. di caffè, un e. di mortadella; formaggio a 2000 lire l'etto''"</ref> |
|||
* The unit name “megagram” is rarely used, and even then, typically only in technical fields in contexts where especially rigorous consistency with the units of measure is desired. For most purposes, the unit “[[tonne]]” is instead used. The tonne and its symbol, t, were adopted by the CIPM in 1879. It is a non-SI unit accepted by the BIPM for use with the SI. In English speaking countries it is usually called “metric ton”.<ref>BIPM: SI Brochure: Section 4.1, ''Non-SI units accepted for use with the SI, and units based on fundamental {{nowrap|constants:'' [http://www.bipm.org/en/si/si_brochure/chapter4/table6.html Table 6]}}<sub> </sub></ref> Note also that the unit name “megatonne” or “megaton” (Mt) is often used in general-interest literature on [[greenhouse gas]] emissions whereas the equivalent value in scientific papers on the subject is often the “teragram” (Tg). |
|||
* The former standard spelling and abbreviation "deka-" and "dk" produced abbreviations such as "dkm" (dekametre) and "dkg" (dekagram).<ref>U.S. National Bureau of Standards, ''The International Metric System of Weights and Measures'', "Official Abbreviations of International Metric Units", 1932, [https://books.google.com/books?id=ujwM_fwA9lEC&pg=PA13 p. 13]</ref> {{As of|2020|post=,}} the abbreviation "dkg" (10 g) is still used in parts of central Europe in retail for some foods such as cheese and meat.<ref name="Czeck dkg ham">{{cite web |title=Jestřebická hovězí šunka 10 dkg {{!}} Rancherské speciality |url=https://eshop.rancherskespeciality.cz/Jestrebicka-hovezi-sunka-10-dkg-d189.htm |website=eshop.rancherskespeciality.cz |language=cs|access-date=June 16, 2020|archive-url=https://web.archive.org/web/20200616032253/https://eshop.rancherskespeciality.cz/Jestrebicka-hovezi-sunka-10-dkg-d189.htm|archive-date=June 16, 2020}}</ref><ref name="Slovak dkg ham">{{cite web |title=Sedliacka šunka 1 dkg {{!}} Gazdovský dvor – Farma Busov Gaboltov |url=http://farmabusoveshop.sk/sedliacka-sunka-1-dkg |website=Sedliacka šunka 1 dkg |language=sk|access-date=June 16, 2020|archive-url=https://web.archive.org/web/20200616033111/http://farmabusoveshop.sk/sedliacka-sunka-1-dkg|archive-date=June 16, 2020}}</ref><ref name="Czech dkg cheese">{{cite web |title=sýr bazalkový – Farmářské Trhy |url=http://www.e-farmarsketrhy.cz/syry-z-kravskeho-mleka/syr-bazalkovy |website=www.e-farmarsketrhy.cz |language=cs|access-date=June 16, 2020|archive-url=https://web.archive.org/web/20200616033522/http://www.e-farmarsketrhy.cz/syry-z-kravskeho-mleka/syr-bazalkovy|archive-date=June 16, 2020}}</ref><ref name="Hungarian menu">{{cite web |title=English Menu – Cafe Mediterran |url=http://www.mediterrangrill.hu/english-menu/ |language=en|access-date=June 16, 2020|archive-url=https://web.archive.org/web/20200616034445/http://www.mediterrangrill.hu/english-menu/|archive-date=June 16, 2020|quote= Beef steak 20 dkg; Beef steak 40 dkg;Thick crust 35 dkg}}</ref><ref name="Hungarian dkg cheese">{{cite web |title=Termékek – Csíz Sajtműhely |url=https://csizsajtmuhely.hu/sajtrendeles/ |language=hu|access-date=June 16, 2020|archive-url=https://web.archive.org/web/20200616035724/https://csizsajtmuhely.hu/sajtrendeles/|archive-date=June 16, 2020}}</ref> |
|||
* The unit name ''megagram'' is rarely used, and even then typically only in technical fields in contexts where especially rigorous consistency with the SI standard is desired. For most purposes, the name ''[[tonne]]'' is instead used. The tonne and its symbol, "t", were adopted by the CIPM in 1879. It is a non-SI unit accepted by the BIPM for use with the SI. According to the BIPM, "This unit is sometimes referred to as 'metric ton' in some English-speaking countries."<ref>''Non-SI units that are accepted for use with the SI'', [https://www.bipm.org/en/publications/si-brochure/ SI Brochure: Section 4 (Table 8)], BIPM</ref> |
|||
==Glossary==<!--NOTE TO EDITORS: This section is not intended to be all-inclusive. It comprises only those words and terms with very specific and/or unique meanings in the discipline of mass metrology. NOTE TOO THAT THIS GLOSSARY IS INTERNALLY LINKED TO FROM MANY PLACES WITHIN THIS ARTICLE. |
|||
--> |
|||
* '''Abstracted''': Isolated and its effect changed in form, often simplified or made more accessible in the process. |
|||
* '''Artifact''': A human-made object used as a comparative standard in the measurement of a physical quantity. |
|||
* '''Check standard''': |
|||
:# A standard body’s backup replica of the International Prototype Kilogram (IPK). |
|||
:# A secondary kilogram mass standard used as a stand-in for the primary standard during routine calibrations. |
|||
* '''Definition''': A formal, specific, and exact specification. |
|||
* '''Delineation''': The physical means used to mark a boundary or express the magnitude of an entity. |
|||
* '''Disseminate''': To widely distribute the magnitude of a unit of measure, typically via replicas and transfer standards. |
|||
* '''IPK''': Abbreviation of “International Prototype Kilogram” ([[:Image:CGKilogram.jpg|CG image]]), the artifact which has a mass defined as precisely one kilogram. |
|||
* '''Magnitude''': The extent or numeric value of a property |
|||
* '''National prototype''': A replica of the IPK possessed by a nation. |
|||
* '''Practical realization''': An artifact or readily reproducible apparatus to conveniently delineate the magnitude of a unit of measure. |
|||
* '''Primary national standard''': |
|||
:# A replica of the IPK possessed by a nation |
|||
:# The least used replica of the IPK when a nation possesses more than one. |
|||
* '''Prototype''': |
|||
:# A human-made object that serves as the defining comparative standard in the measurement of a physical quantity. |
|||
:# A human-made object that serves as ''the'' comparative standard in the measurement of a physical quantity. |
|||
:# The IPK and any of its replicas |
|||
* '''Replica''': An official copy of the IPK. |
|||
* '''Sister copy''': One of six official copies of the IPK that are stored in the same safe as the IPK and are used as check standards by the BIPM. |
|||
* '''Transfer standard''': An artifact or apparatus that reproduces the magnitude of a unit of measure in a different, usually more practical, form. |
|||
== See also == |
== See also == |
||
{{ |
{{Portal|Physics}} |
||
* {{Annotated link|Inertia}} |
|||
{{col-break}} |
|||
* {{Annotated link|Kibble balance}} |
|||
* [[Inertia]] |
|||
* {{Annotated link|Kilogram-force}} |
|||
* [[International System of Units]] (SI) |
|||
* {{Annotated link|Mass versus weight}} |
|||
* [[International Bureau of Weights and Measures]] (BIPM) |
|||
* {{Annotated link|Metric system}} |
|||
* [[International Committee for Weights and Measures]] (CIPM) |
|||
* {{Annotated link|National Institute of Standards and Technology}} (NIST) |
|||
* [[General Conference on Weights and Measures]] (CGPM) |
|||
* {{Annotated link|Newton (unit)|Newton}} |
|||
* [[Gram]] |
|||
* {{Annotated link|Standard gravity}} |
|||
* [[Grave (mass)|Grave]] (orig. name of the kilogram, history of) |
|||
* {{Annotated link|Weight}} |
|||
* [[Gravimetry]] |
|||
* [[Kilogram-force]] |
|||
== Notes == |
|||
* [[Metric system]] |
|||
<references group="Note" /> |
|||
{{col-break}} |
|||
* [[Mass]] |
|||
* [[Mass versus weight]] |
|||
* [[National Institute of Standards and Technology]] (NIST) |
|||
* [[Newton]] |
|||
* [[SI base unit]]s |
|||
* [[Standard gravity]] |
|||
* [[Tonne]] (metric ton) |
|||
* [[Watt balance]] |
|||
* [[Weight]] |
|||
{{col-end}} |
|||
== |
== References == |
||
{{Reflist|30em}} |
|||
<references/> |
|||
==External links== |
== External links == |
||
{{Commons category}} |
|||
{{externalimage |
|||
{{External media |
|||
|align=right |
|||
| float = right |
|||
|width=40% |
|||
| width = 40% |
|||
|image1=BIPM: [http://www.bipm.org/en/scientific/mass/pictures_mass/prototype.html The IPK in three nested bell jars] |
|||
|image2=NIST: [http:// |
| image2 = NIST: [http://patapsco.nist.gov/imagegallery/retrieve.cfm?imageid=49&dpi=72&fileformat=jpg K20, the US National Prototype Kilogram] resting on an egg crate fluorescent light panel |
||
|image3=BIPM: ''[http://www.bipm.org/en/scientific/mass/pictures_mass/cleaning.html Steam cleaning a 1 |
| image3 = BIPM: ''[https://web.archive.org/web/20070926215612/http://www.bipm.org/en/scientific/mass/pictures_mass/cleaning.html Steam cleaning a 1 kg prototype before a mass comparison]'' |
||
|image4=BIPM: [http://www.bipm.org/en/scientific/mass/pictures_mass/vault.html The IPK and its six sister copies in their vault] |
| image4 = BIPM: [https://web.archive.org/web/20070926221138/http://www.bipm.org/en/scientific/mass/pictures_mass/vault.html The IPK and its six sister copies in their vault] |
||
|image5=The Age: [http://www.theage.com.au/ffximage/2007/06/14/rgN1506_csiro_wideweb__470x343,0.jpg Silicon sphere for the Avogadro Project] |
| image5 = The Age: [http://www.theage.com.au/ffximage/2007/06/14/rgN1506_csiro_wideweb__470x343,0.jpg Silicon sphere for the Avogadro Project] |
||
|image6=NPL: [http://www.npl.co.uk/ |
| image6 = NPL: [http://www.npl.co.uk/content/conMediaFile/1083 The NPL's Watt Balance project] |
||
|image7=NIST: This particular [http://museum.nist.gov/object.asp?ObjID=51 Rueprecht Balance], an Austrian-made precision balance, was used by the NIST from 1945 until 1960 |
| image7 = NIST: This particular [http://museum.nist.gov/object.asp?ObjID=51 Rueprecht Balance], an Austrian-made precision balance, was used by the NIST from 1945 until 1960 |
||
|image8=BIPM: [http://www.bipm.org/en/scientific/mass/research_mass/flexure-strip.html The FB |
| image8 = BIPM: [https://web.archive.org/web/20070926215949/http://www.bipm.org/en/scientific/mass/research_mass/flexure-strip.html The FB{{nbhyph}}2 flexure-strip balance], the BIPM's modern precision balance featuring a standard deviation of one ten-billionth of a kilogram (0.1{{nbsp}}μg) |
||
|image9=BIPM: [http://www.bipm.org/en/scientific/mass/pictures_mass/mettler.html Mettler HK1000 balance], featuring 1 |
| image9 = BIPM: [https://web.archive.org/web/20070926220910/http://www.bipm.org/en/scientific/mass/pictures_mass/mettler.html Mettler HK1000 balance], featuring 1{{nbsp}}μg resolution and a 4{{nbsp}}kg maximum mass. Also used by NIST and Sandia National Laboratories' Primary Standards Laboratory |
||
|image10=Micro-g LaCoste: [http://www.microglacoste.com/images/FG5.small.jpg FG |
| image10 = Micro-g LaCoste: [http://www.microglacoste.com/images/FG5.small.jpg FG{{nbhyph}}5 absolute gravimeter], ([http://www.microglacoste.com/images/fg5schem.jpg diagram]), used in national laboratories to measure gravity to 2{{nbsp}}[[Gal (acceleration)|μGal]]<!--NOTE TO EDITORS: The Micro-g LaCoste Web site does not display properly on some computer systems. The accuracy is NOT 2 mGal and is indeed 2 μGal. Please see discussion topic at [[Talk:Kilogram#Micro-g_LaCoste_FG-5_accuracy]]--> accuracy |
||
}} |
}} |
||
* |
* [https://www.nist.gov/public_affairs/releases/electrokilogram.cfm NIST Improves Accuracy of 'Watt Balance' Method for Defining the Kilogram] |
||
* The |
* The UK's National Physical Laboratory (NPL): [<!-- http://www.npl.co.uk/server.php?show=ConWebDoc.2087 -->https://web.archive.org/web/20160323212037/http://www.npl.co.uk/reference/faqs/are-any-problems-caused-by-having-the-kilogram-defined-in-terms-of-a-physical-artefact-(faq-mass-and-density) <!-- An overview of the problems with an artefact-based kilogram -->Are any problems caused by having the kilogram defined in terms of a physical artefact? (FAQ – Mass & Density)] |
||
* NPL: ''[http://www.npl.co.uk/ |
* NPL: ''[http://www.npl.co.uk/science-technology/mass-and-force/research/npl-kibble-balance NPL Kibble balance]'' |
||
* |
* Metrology in France: ''[http://www.french-metrology.com/en/feature/watt-balance.asp Watt balance]'' |
||
* Australian National Measurement Institute: ''[http://www.measurement.gov.au/SCIENCETECHNOLOGY/Pages/MassandRelatedQuantities.aspx Redefining the kilogram through the Avogadro constant]'' |
|||
* Metrology in France: ''[http://www.metrologiefrancaise.com/en/feature/watt-balance.asp Watt balance]'' |
|||
* Australian National Measurement Institute: ''[http://www.measurement.gov.au/index.cfm?event=object.showContent&objectID=7CBA5E62-BCD6-81AC-15017BE81748EF4B Redefining the kilogram through the Avogadro constant]'' |
|||
* International Bureau of Weights and Measures (BIPM): [http://www.bipm.org/en/home/ Home page] |
* International Bureau of Weights and Measures (BIPM): [http://www.bipm.org/en/home/ Home page] |
||
* NZZ Folio: ''[http://www.nzzfolio.ch/www/d80bd71b-b264-4db4-afd0-277884b93470/showarticle/fb0ba22e-46b7-43a5-8320-ef16483b7e91.aspx What a kilogram really weighs]'' |
* NZZ Folio: ''[http://www.nzzfolio.ch/www/d80bd71b-b264-4db4-afd0-277884b93470/showarticle/fb0ba22e-46b7-43a5-8320-ef16483b7e91.aspx What a kilogram really weighs]'' |
||
* NPL: ''[http://www.npl.co.uk/server.php?show=ConWebDoc.1380 What are the differences between mass, weight, force and load?]'' |
* NPL: ''[<!-- http://www.npl.co.uk/server.php?show=ConWebDoc.1380 -->http://www.npl.co.uk/reference/faqs/what-are-the-differences-between-mass,-weight,-force-and-load-(faq-mass-and-density) What are the differences between mass, weight, force and load?]'' |
||
* BBC: ''[http://news.bbc.co.uk/ |
* BBC: ''[http://news.bbc.co.uk/2/hi/science/nature/7084099.stm Getting the measure of a kilogram]'' |
||
* NPR: ''[https://www.npr.org/templates/story/story.php?storyId=112003322 This Kilogram Has A Weight-Loss Problem]'', an interview with [[National Institute of Standards and Technology]] physicist Richard Steiner |
|||
* [https://web.archive.org/web/20110717124427/http://www.inrim.it/Nah/Web_Nah/home.htm Avogadro and molar Planck constants for the redefinition of the kilogram] |
|||
* [http://arquivo.pt/wayback/20160513163237/http://www.inrim.it/know/ Realization of the awaited definition of the kilogram] |
|||
* {{cite news |url=https://www.theguardian.com/science/2018/nov/09/in-the-balance-scientists-vote-on-first-change-to-kilogram-in-century |title=In the balance: scientists vote on first change to kilogram in a century |newspaper=[[The Guardian]] |first=Ian |last=Sample |date=November 9, 2018 |access-date=November 9, 2018}} |
|||
=== Videos === |
|||
* [https://www.youtube.com/thebipm The BIPM] – [[YouTube]] channel |
|||
* [https://www.youtube.com/watch?v=yhgb23tAFFs&list=PL-vj-3_a7wTDeKEupZSX7Tw42yReNgJLl "The role of the Planck constant in physics" – presentation at 26th CGPM meeting at Versailles, France, November 2018 when voting on superseding the IPK took place] on [[YouTube]] |
|||
{{SI units}} |
|||
{{Authority control}} |
|||
{{Good article}} |
|||
[[Category:SI base units]] |
[[Category:SI base units]] |
||
[[Category:Units of mass]] |
[[Category:Units of mass]] |
||
[[Category:1000 (number)]] |
|||
[[als:Kilogramm]] |
|||
[[ar:كيلوغرام]] |
|||
[[ay:Kilu]] |
|||
[[bn:কিলোগ্রাম]] |
|||
[[zh-min-nan:Kong-kin]] |
|||
[[be:Кілаграм]] |
|||
[[be-x-old:Кіляграм]] |
|||
[[bs:Kilogram]] |
|||
[[br:Kilogram]] |
|||
[[bg:Килограм]] |
|||
[[ca:Quilogram]] |
|||
[[cs:Kilogram]] |
|||
[[da:Kilogram]] |
|||
[[de:Kilogramm]] |
|||
[[et:Kilogramm]] |
|||
[[el:Χιλιόγραμμο]] |
|||
[[es:Kilogramo]] |
|||
[[eo:Kilogramo]] |
|||
[[eu:Kilogramo]] |
|||
[[fa:کیلوگرم]] |
|||
[[fr:Kilogramme]] |
|||
[[fur:Chilogram]] |
|||
[[ga:Cileagram]] |
|||
[[gan:公斤]] |
|||
[[gl:Quilogramo]] |
|||
[[ko:킬로그램]] |
|||
[[hi:किलोग्राम]] |
|||
[[hr:Kilogram]] |
|||
[[id:Kilogram]] |
|||
[[ia:Kilogramma]] |
|||
[[is:Kílógramm]] |
|||
[[it:Chilogrammo]] |
|||
[[he:קילוגרם]] |
|||
[[jv:Kilogram]] |
|||
[[ka:კილოგრამი]] |
|||
[[sw:Kilogramu]] |
|||
[[la:Chiliogramma]] |
|||
[[lv:Kilograms]] |
|||
[[lb:Kilogramm]] |
|||
[[lt:Kilogramas]] |
|||
[[ln:Kilogálame]] |
|||
[[hu:Kilogramm]] |
|||
[[mk:Килограм]] |
|||
[[ml:കിലോഗ്രാം]] |
|||
[[mr:किलोग्रॅम]] |
|||
[[ms:Kilogram]] |
|||
[[mn:Килограмм]] |
|||
[[nl:Kilogram]] |
|||
[[ja:キログラム]] |
|||
[[no:Kilogram]] |
|||
[[nn:Kilogram]] |
|||
[[uz:Kilogramm]] |
|||
[[pl:Kilogram]] |
|||
[[pt:Quilograma]] |
|||
[[ksh:Masse]] |
|||
[[ro:Kilogram]] |
|||
[[ru:Килограмм]] |
|||
[[sco:Kilogram]] |
|||
[[sq:Kilogrami]] |
|||
[[scn:Chilugrammu]] |
|||
[[simple:Kilogram]] |
|||
[[sk:Kilogram]] |
|||
[[sl:Kilogram]] |
|||
[[sr:Килограм]] |
|||
[[sh:Kilogram]] |
|||
[[su:Kilogram]] |
|||
[[fi:Kilogramma]] |
|||
[[sv:Kilogram]] |
|||
[[ta:கிலோகிராம்]] |
|||
[[th:กิโลกรัม]] |
|||
[[vi:Kilôgam]] |
|||
[[uk:Кілограм]] |
|||
[[ur:کلوگرام]] |
|||
[[yi:קילאגראם]] |
|||
[[zh-yue:千克]] |
|||
[[bat-smg:Kėluograms]] |
|||
[[zh:千克]] |
|||
Latest revision as of 15:37, 5 June 2024
| kilogram | |
|---|---|
 A series of 5, 2, 1, 0.5 and 0.2 kilogram weights, made out of rusty cast iron | |
| General information | |
| Unit system | SI |
| Unit of | mass |
| Symbol | kg |
| Conversions | |
| 1 kg in ... | ... is equal to ... |
| Avoirdupois | ≈ 2.204623 pounds[Note 1] |
| British Gravitational | ≈ 0.0685 slugs |
| CGS units | 1000 grams |
| Atomic mass units | 6.02214076×1026 Da |
The kilogram (also kilogramme[1]) is the base unit of mass in the International System of Units (SI), having the unit symbol kg. It is a widely used measure in science, engineering and commerce worldwide, and is often simply called a kilo colloquially.[2] It means 'one thousand grams'.
The kilogram is a SI base unit, defined in terms of two other base units, the second and the metre and the Planck constant, a SI defining constant.[3]: 131 A properly equipped metrology laboratory can calibrate a mass measurement instrument such as a Kibble balance as a primary standard for the kilogram mass.[4]
The kilogram was originally defined in 1795 during the French Revolution as the mass of one litre of water. The current definition of a kilogram agrees with this original definition to within 30 parts per million. In 1799, the platinum Kilogramme des Archives replaced it as the standard of mass. In 1889, a cylinder of platinum-iridium, the International Prototype of the Kilogram (IPK), became the standard of the unit of mass for the metric system and remained so for 130 years, before the current standard was adopted in 2019.[5]
Definition[edit]
The kilogram is defined in terms of three defining constants:[3]
- a specific atomic transition frequency ΔνCs, which defines the duration of the second,
- the speed of light c, which when combined with the second, defines the length of the metre,
- and the Planck constant h, which when combined with the metre and second, defines the mass of the kilogram.
The formal definition according to the General Conference on Weights and Measures (CGPM) is:
The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h to be 6.62607015×10−34 when expressed in the unit J⋅s, which is equal to kg⋅m2⋅s−1, where the metre and the second are defined in terms of c and ΔνCs.
Defined in term of those units, the kg is formulated as:[8]
- kg = (299792458)2/(6.62607015×10−34)(9192631770)hΔνCs/c2 = 917097121160018/621541050725904751042hΔνCs/c2 ≈ (1.475521399735270×1040)hΔνCs/c2 .
This definition is generally consistent with previous definitions: the mass remains within 30 ppm of the mass of one litre of water.[9]
Timeline of previous definitions[edit]

- 1793: The grave (the precursor of the kilogram) was defined as the mass of 1 litre (dm3) of water, which was determined to be 18841 grains.[10]
- 1795: the gram (1/1000 of a kilogram) was provisionally defined as the mass of one cubic centimetre of water at the melting point of ice.[11]
- 1799: The Kilogramme des Archives was manufactured as a prototype. It had a mass equal to the mass of 1 dm3 of water at the temperature of its maximum density, which is approximately 4 °C.[12]
- 1875–1889: The Metre Convention was signed in 1875, leading to the production of the International Prototype of the Kilogram (IPK) in 1879 and its adoption in 1889.[13]
- 2019: The kilogram was defined in terms of the Planck constant, the speed of light and hyperfine transition frequency of 133Cs as approved by the General Conference on Weights and Measures (CGPM) on November 16, 2018.[5]
Name and terminology[edit]
The kilogram is the only base SI unit with an SI prefix (kilo) as part of its name. The word kilogramme or kilogram is derived from the French kilogramme,[14] which itself was a learned coinage, prefixing the Greek stem of χίλιοι khilioi "a thousand" to gramma, a Late Latin term for "a small weight", itself from Greek γράμμα.[15] The word kilogramme was written into French law in 1795, in the Decree of 18 Germinal,[16] which revised the provisional system of units introduced by the French National Convention two years earlier, where the gravet had been defined as weight (poids) of a cubic centimetre of water, equal to 1/1000 of a grave.[17] In the decree of 1795, the term gramme thus replaced gravet, and kilogramme replaced grave.[12]
The French spelling was adopted in Great Britain when the word was used for the first time in English in 1795,[18][14] with the spelling kilogram being adopted in the United States. In the United Kingdom both spellings are used, with "kilogram" having become by far the more common.[1] UK law regulating the units to be used when trading by weight or measure does not prevent the use of either spelling.[19]
In the 19th century the French word kilo, a shortening of kilogramme, was imported into the English language where it has been used to mean both kilogram[20] and kilometre[citation needed].[21] While kilo as an alternative is acceptable, to The Economist for example,[22] the Canadian government's Termium Plus system states that "SI (International System of Units) usage, followed in scientific and technical writing" does not allow its usage and it is described as "a common informal name" on Russ Rowlett's Dictionary of Units of Measurement.[23][24] When the United States Congress gave the metric system legal status in 1866, it permitted the use of the word kilo as an alternative to the word kilogram,[25] but in 1990 revoked the status of the word kilo.[26]
The SI system was introduced in 1960 and in 1970 the BIPM started publishing the SI Brochure, which contains all relevant decisions and recommendations by the CGPM concerning units. The SI Brochure states that "It is not permissible to use abbreviations for unit symbols or unit names ...".[27][Note 2]
For use with east Asian character sets, the SI symbol is encoded as a single Unicode character, U+338F ㎏ SQUARE KG in the CJK Compatibility block.
Redefinition based on fundamental constants[edit]
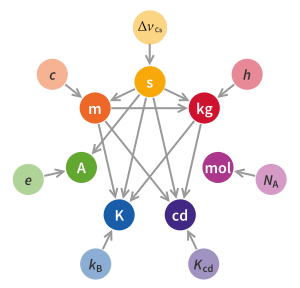

The replacement of the International Prototype of the Kilogram (IPK) as the primary standard was motivated by evidence accumulated over a long period of time that the mass of the IPK and its replicas had been changing; the IPK had diverged from its replicas by approximately 50 micrograms since their manufacture late in the 19th century. This led to several competing efforts to develop measurement technology precise enough to warrant replacing the kilogram artefact with a definition based directly on physical fundamental constants.[5]
The International Committee for Weights and Measures (CIPM) approved a redefinition of the SI base units in November 2018 that defines the kilogram by defining the Planck constant to be exactly 6.62607015×10−34 kg⋅m2⋅s−1, effectively defining the kilogram in terms of the second and the metre. The new definition took effect on May 20, 2019.[5][6][28]
Prior to the redefinition, the kilogram and several other SI units based on the kilogram were defined by a man-made metal artifact: the Kilogramme des Archives from 1799 to 1889, and the IPK from 1889 to 2019.[5]
In 1960, the metre, previously similarly having been defined with reference to a single platinum-iridium bar with two marks on it, was redefined in terms of an invariant physical constant (the wavelength of a particular emission of light emitted by krypton,[29] and later the speed of light) so that the standard can be independently reproduced in different laboratories by following a written specification.
At the 94th Meeting of the CIPM in 2005, it was recommended that the same be done with the kilogram.[30]
In October 2010, the CIPM voted to submit a resolution for consideration at the General Conference on Weights and Measures (CGPM), to "take note of an intention" that the kilogram be defined in terms of the Planck constant, h (which has dimensions of energy times time, thus mass × length2 / time) together with other physical constants.[31][32] This resolution was accepted by the 24th conference of the CGPM[33] in October 2011 and further discussed at the 25th conference in 2014.[34][35] Although the Committee recognised that significant progress had been made, they concluded that the data did not yet appear sufficiently robust to adopt the revised definition, and that work should continue to enable the adoption at the 26th meeting, scheduled for 2018.[34] Such a definition would theoretically permit any apparatus that was capable of delineating the kilogram in terms of the Planck constant to be used as long as it possessed sufficient precision, accuracy and stability. The Kibble balance is one way to do this.[36]
As part of this project, a variety of very different technologies and approaches were considered and explored over many years. Some of these approaches were based on equipment and procedures that would enable the reproducible production of new, kilogram-mass prototypes on demand (albeit with extraordinary effort) using measurement techniques and material properties that are ultimately based on, or traceable to, physical constants. Others were based on devices that measured either the acceleration or weight of hand-tuned kilogram test masses and which expressed their magnitudes in electrical terms via special components that permit traceability to physical constants. All approaches depend on converting a weight measurement to a mass and therefore require precise measurement of the strength of gravity in laboratories (gravimetry). All approaches would have precisely fixed one or more constants of nature at a defined value.[citation needed]
SI multiples[edit]
Because an SI unit may not have multiple prefixes (see SI prefix), prefixes are added to gram, rather than the base unit kilogram, which already has a prefix as part of its name.[37] For instance, one-millionth of a kilogram is 1 mg (one milligram), not 1 μkg (one microkilogram).
| Submultiples | Multiples | ||||
|---|---|---|---|---|---|
| Value | SI symbol | Name | Value | SI symbol | Name |
| 10−1 g | dg | decigram | 101 g | dag | decagram |
| 10−2 g | cg | centigram | 102 g | hg | hectogram |
| 10−3 g | mg | milligram | 103 g | kg | kilogram |
| 10−6 g | μg | microgram | 106 g | Mg | megagram (tonne) |
| 10−9 g | ng | nanogram | 109 g | Gg | gigagram |
| 10−12 g | pg | picogram | 1012 g | Tg | teragram |
| 10−15 g | fg | femtogram | 1015 g | Pg | petagram |
| 10−18 g | ag | attogram | 1018 g | Eg | exagram |
| 10−21 g | zg | zeptogram | 1021 g | Zg | zettagram |
| 10−24 g | yg | yoctogram | 1024 g | Yg | yottagram |
| 10−27 g | rg | rontogram | 1027 g | Rg | ronnagram |
| 10−30 g | qg | quectogram | 1030 g | Qg | quettagram |
| Common prefixed units are in bold face.[Note 3] | |||||
Practical issues with SI weight names[edit]
- Serious medication errors have been made by confusing milligrams and micrograms when micrograms has been abbreviated.[38] The abbreviation "mcg" rather than the SI symbol "μg" is formally mandated for medical practitioners in the US by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO).[39] In the United Kingdom, the National Institute for Health and Care Excellence and Scottish Palliative Care Guidelines state that "micrograms" and "nanograms" must both be written in full, and never abbreviated as "mcg" or "μg".[38][40]
- The hectogram (100 g) (Italian: ettogrammo or etto) is a very commonly used unit in the retail food trade in Italy.[41][42][43]
- The former standard spelling and abbreviation "deka-" and "dk" produced abbreviations such as "dkm" (dekametre) and "dkg" (dekagram).[44] As of 2020,[update] the abbreviation "dkg" (10 g) is still used in parts of central Europe in retail for some foods such as cheese and meat.[45][46][47][48][49]
- The unit name megagram is rarely used, and even then typically only in technical fields in contexts where especially rigorous consistency with the SI standard is desired. For most purposes, the name tonne is instead used. The tonne and its symbol, "t", were adopted by the CIPM in 1879. It is a non-SI unit accepted by the BIPM for use with the SI. According to the BIPM, "This unit is sometimes referred to as 'metric ton' in some English-speaking countries."[50]
See also[edit]
- Inertia – Fundamental principle of classical physics
- Kibble balance – Electromechanical weight measuring instrument
- Kilogram-force – Weight on earth of a one-kilogram mass
- Mass versus weight – Distinction between mass and weight
- Metric system – Metre-based systems of measurement
- National Institute of Standards and Technology – Measurement standards laboratory in the United States (NIST)
- Newton – Unit of force in physics
- Standard gravity – Standard gravitational acceleration on Earth
- Weight – Force on a mass due to gravity
Notes[edit]
- ^ The avoirdupois pound is part of both United States customary system of units and the Imperial system of units. It is defined as exactly 0.45359237 kilograms.
- ^ The French text (which is the authoritative text) states "Il n'est pas autorisé d'utiliser des abréviations pour les symboles et noms d'unités ..."
- ^ Criterion: A combined total of at least five occurrences on the British National Corpus and the Corpus of Contemporary American English, including both the singular and the plural for both the -gram and the -gramme spelling.
References[edit]
- ^ a b "Kilogram". Oxford Dictionaries. Archived from the original on January 31, 2013. Retrieved November 3, 2011.
- ^ Merriam-Mebster definition of Kilo
- ^ a b International Bureau of Weights and Measures (May 20, 2019), The International System of Units (SI) (PDF) (9th ed.), ISBN 978-92-822-2272-0, archived from the original on October 18, 2021
- ^ "Mise en pratique for the definition of the kilogram in the SI". BIPM.org. July 7, 2021. Retrieved February 18, 2022.
- ^ a b c d e Resnick, Brian (May 20, 2019). "The new kilogram just debuted. It's a massive achievement". vox.com. Retrieved May 23, 2019.
- ^ a b Draft Resolution A "On the revision of the International System of units (SI)" to be submitted to the CGPM at its 26th meeting (2018) (PDF), archived (PDF) from the original on April 2, 2021
- ^ Decision CIPM/105-13 (October 2016). The day is the 144th anniversary of the Metre Convention.
- ^ SI Brochure: The International System of Units (SI). BIPM, 9th edition, 2019.
- ^ The density of water is 0.999972 g/cm3 at 3.984 °C. See Franks, Felix (2012). The Physics and Physical Chemistry of Water. Springer. ISBN 978-1-4684-8334-5.
- ^ Guyton; Lavoisier; Monge; Berthollet; et al. (1792). Annales de chimie ou Recueil de mémoires concernant la chimie et les arts qui en dépendent. Vol. 15–16. Paris: Chez Joseph de Boffe. p. 277.
- ^ Gramme, le poids absolu d'un volume d'eau pure égal au cube de la centième partie du mètre, et à la température de la glace fondante
- ^ a b Zupko, Ronald Edward (1990). Revolution in Measurement: Western European Weights and Measures Since the Age of Science. Philadelphia: American Philosophical Society. ISBN 978-0-87169-186-6.
- ^ "Treaty of the Metre". Encyclopædia Britannica. 2023. Retrieved July 18, 2023.
- ^ a b "Kilogram". Oxford English Dictionary. Oxford University Press. Retrieved November 3, 2011.
- ^ Fowlers, HW; Fowler, FG (1964). The Concise Oxford Dictionary. Oxford: The Clarendon Press. Greek γράμμα (as it were γράφ-μα, Doric γράθμα) means "something written, a letter", but it came to be used as a unit of weight, apparently equal to 1/24 of an ounce (1/288 of a libra, which would correspond to about 1.14 grams in modern units), at some time during Late Antiquity. French gramme was adopted from Latin gramma, itself quite obscure, but found in the Carmen de ponderibus et mensuris (8.25) attributed by Remmius Palaemon (fl. 1st century), where it is the weight of two oboli (Charlton T. Lewis, Charles Short, A Latin Dictionary s.v. "gramma", 1879). Henry George Liddell. Robert Scott. A Greek-English Lexicon (revised and augmented edition, Oxford, 1940) s.v. γράμμα, citing the 10th-century work Geoponica and a 4th-century papyrus edited in L. Mitteis, Griechische Urkunden der Papyrussammlung zu Leipzig, vol. i (1906), 62 ii 27.
- ^ "Décret relatif aux poids et aux mesures du 18 germinal an 3 (7 avril 1795)" [Decree of 18 Germinal, year III (April 7, 1795) regarding weights and measures]. Grandes lois de la République (in French). Digithèque de matériaux juridiques et politiques, Université de Perpignan. Retrieved November 3, 2011.
- ^ Convention nationale, décret du 1er août 1793, ed. Duvergier, Collection complète des lois, décrets, ordonnances, règlemens avis du Conseil d'état, publiée sur les éditions officielles du Louvre, vol. 6 (2nd ed. 1834), p. 70. The metre (mètre) on which this definition depends was itself defined as the ten-millionth part of a quarter of Earth's meridian, given in traditional units as 3 pieds, 11.44 lignes (a ligne being the 12th part of a pouce (inch), or the 144th part of a pied.
- ^ Peltier, Jean-Gabriel (1795). "Paris, during the year 1795". Monthly Review. 17: 556. Retrieved August 2, 2018. Contemporaneous English translation of the French decree of 1795
- ^ "Spelling of "gram", etc". Weights and Measures Act 1985. Her Majesty's Stationery Office. October 30, 1985. Retrieved November 6, 2011.
- ^ "kilo (n1)". Oxford English Dictionary (2nd ed.). Oxford: Oxford University Press. 1989. Retrieved November 8, 2011.
- ^ "kilo (n2)". Oxford English Dictionary (2nd ed.). Oxford: Oxford University Press. 1989. Retrieved November 8, 2011.
- ^ "Style Guide" (PDF). The Economist. January 7, 2002. Archived from the original (PDF) on July 1, 2017. Retrieved November 8, 2011.
- ^ "kilogram, kg, kilo". Termium Plus. Government of Canada. October 8, 2009. Retrieved May 29, 2019.
- ^ "kilo". How Many?. Archived from the original on November 16, 2011. Retrieved November 6, 2011.
- ^
29th Congress of the United States, Session 1 (May 13, 1866). "H.R. 596, An Act to authorize the use of the metric system of weights and measures". Archived from the original on July 5, 2015.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^
"Metric System of Measurement:Interpretation of the International System of Units for the United States; Notice" (PDF). Federal Register. 63 (144): 40340. July 28, 1998. Archived from the original (PDF) on October 15, 2011. Retrieved November 10, 2011.
Obsolete Units As stated in the 1990 Federal Register notice, ...
- ^ International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), p. 130, ISBN 92-822-2213-6, archived (PDF) from the original on June 4, 2021, retrieved December 16, 2021
- ^ Pallab Ghosh (November 16, 2018). "Kilogram gets a new definition". BBC News. Retrieved November 16, 2018.
- ^ International Bureau of Weights and Measures (2006), The International System of Units (SI) (PDF) (8th ed.), p. 112, ISBN 92-822-2213-6, archived (PDF) from the original on June 4, 2021, retrieved December 16, 2021
- ^ Recommendation 1: Preparative steps towards new definitions of the kilogram, the ampere, the kelvin and the mole in terms of fundamental constants (PDF). 94th meeting of the International Committee for Weights and Measures. October 2005. p. 233. Archived (PDF) from the original on June 30, 2007. Retrieved February 7, 2018.
- ^ "NIST Backs Proposal for a Revamped System of Measurement Units". NIST. Nist.gov. October 26, 2010. Retrieved April 3, 2011.
- ^ Ian Mills (September 29, 2010). "Draft Chapter 2 for SI Brochure, following redefinitions of the base units" (PDF). CCU. Retrieved January 1, 2011.
- ^ Resolution 1 – On the possible future revision of the International System of Units, the SI (PDF). 24th meeting of the General Conference on Weights and Measures. Sèvres, France. October 17–21, 2011. Retrieved October 25, 2011.
- ^ a b "BIPM – Resolution 1 of the 25th CGPM". www.bipm.org. Retrieved March 27, 2017.
- ^ "General Conference on Weights and Measures approves possible changes to the International System of Units, including redefinition of the kilogram" (PDF) (Press release). Sèvres, France: General Conference on Weights and Measures. October 23, 2011. Retrieved October 25, 2011.
- ^ Robinson, Ian A.; Schlamminger, Stephan (2016). "The watt or Kibble balance: A technique for implementing the new SI definition of the unit of mass". Metrologia. 53 (5): A46–A74. Bibcode:2016Metro..53A..46R. doi:10.1088/0026-1394/53/5/A46. PMC 8752041. PMID 35023879.
- ^ BIPM: SI Brochure: Section 3.2, The kilogram Archived March 29, 2016, at the Wayback Machine
- ^ a b "Prescribing Information for Liquid Medicines". Scottish Palliative Care Guidelines. Archived from the original on July 10, 2018. Retrieved June 15, 2015.
- ^ "New Joint Commission "Do Not Use" List: Abbreviations, Acronyms, and Symbols". American Academy of Physical Medicine and Rehabilitation. Archived from the original on September 15, 2015. Retrieved February 19, 2024.
- ^ "Prescription writing". National Institute for Health and Care Excellence. Retrieved February 19, 2024.
- ^ Tom Stobart, The Cook's Encyclopedia, 1981, p. 525
- ^ J.J. Kinder, V.M. Savini, Using Italian: A Guide to Contemporary Usage, 2004, ISBN 0521485568, p. 231
- ^ Giacomo Devoto, Gian Carlo Oli, Nuovo vocabolario illustrato della lingua italiana, 1987, s.v. 'ètto': "frequentissima nell'uso comune: un e. di caffè, un e. di mortadella; formaggio a 2000 lire l'etto"
- ^ U.S. National Bureau of Standards, The International Metric System of Weights and Measures, "Official Abbreviations of International Metric Units", 1932, p. 13
- ^ "Jestřebická hovězí šunka 10 dkg | Rancherské speciality". eshop.rancherskespeciality.cz (in Czech). Archived from the original on June 16, 2020. Retrieved June 16, 2020.
- ^ "Sedliacka šunka 1 dkg | Gazdovský dvor – Farma Busov Gaboltov". Sedliacka šunka 1 dkg (in Slovak). Archived from the original on June 16, 2020. Retrieved June 16, 2020.
- ^ "sýr bazalkový – Farmářské Trhy". www.e-farmarsketrhy.cz (in Czech). Archived from the original on June 16, 2020. Retrieved June 16, 2020.
- ^ "Termékek – Csíz Sajtműhely" (in Hungarian). Archived from the original on June 16, 2020. Retrieved June 16, 2020.
- ^ Non-SI units that are accepted for use with the SI, SI Brochure: Section 4 (Table 8), BIPM
External links[edit]
- NIST Improves Accuracy of 'Watt Balance' Method for Defining the Kilogram
- The UK's National Physical Laboratory (NPL): Are any problems caused by having the kilogram defined in terms of a physical artefact? (FAQ – Mass & Density)
- NPL: NPL Kibble balance
- Metrology in France: Watt balance
- Australian National Measurement Institute: Redefining the kilogram through the Avogadro constant
- International Bureau of Weights and Measures (BIPM): Home page
- NZZ Folio: What a kilogram really weighs
- NPL: What are the differences between mass, weight, force and load?
- BBC: Getting the measure of a kilogram
- NPR: This Kilogram Has A Weight-Loss Problem, an interview with National Institute of Standards and Technology physicist Richard Steiner
- Avogadro and molar Planck constants for the redefinition of the kilogram
- Realization of the awaited definition of the kilogram
- Sample, Ian (November 9, 2018). "In the balance: scientists vote on first change to kilogram in a century". The Guardian. Retrieved November 9, 2018.
Videos[edit]
- The BIPM – YouTube channel
- "The role of the Planck constant in physics" – presentation at 26th CGPM meeting at Versailles, France, November 2018 when voting on superseding the IPK took place on YouTube