Wolverine and Glyceraldehyde 3-phosphate dehydrogenase: Difference between pages
m Reverted edits by 71.212.124.13 (talk) to last version by JohnInDC |
|||
| Line 1: | Line 1: | ||
{{ |
{{PBB|geneid=2597}} |
||
{{Taxobox |
|||
<!-- lead section --> |
|||
| name = Wolverine |
|||
'''Glyceraldehyde 3-phosphate dehydrogenase''' (abbreviated as '''GAPDH''' or less commonly as G3PDH) ({{EC number|1.2.1.12}}) is an [[enzyme]] that catalyzes the sixth step of [[glycolysis]] and thus serves to break down [[glucose]] for energy and carbon molecules. In addition to this long established metabolic function, GAPDH has recently been implicated in several non-metabolic processes, including [[Transcription (genetics)|transcription]] activation, initiation of [[apoptosis]] <ref name="pmid17072346">{{cite journal |author= A. Tarze, A. Deniaud, M. Le Bras, E. Maillier, D. Molle, N. Larochette, N. Zamzami, G. Jan, G. Kroemer, and C. Brenner |title= GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization |journal=Oncogene |volume=26 |issue=18 |pages=2606–2620 |year=2007 |pmid= 17072346 |doi= 10.1038/sj.onc.1210074}}</ref> , and [[COPI|ER to Golgi vesicle shuttling]]. |
|||
| status = VU |
|||
<!-- lead section end --> |
|||
| trend = unknown |
|||
| status_system = iucn2.3 |
|||
== Metabolic function == |
|||
| status_ref = <ref name="iucn">{{IUCN2006|assessors=Mustelid Specialist Group|year=1996|id=9561|title=Gulo gulo|downloaded=11 May 2006}} Listed as Vulnerable (VU A2c v2.3).</ref> |
|||
| image = Gulo gulo 2.jpg |
|||
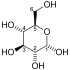
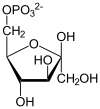
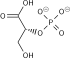
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) catalyses the conversion of [[glyceraldehyde 3-phosphate]] as the name indicates. This is the 6th step of the breakdown of glucose ([[glycolysis]]), an important pathway of energy and carbon molecule supply located in the [[cytosol]] of eukaryotic cells. Glyceraldehyde 3-phosphate is converted to <small>D</small>-[[glycerate 1,3-bisphosphate]] in two coupled steps. The first is favourable and allows the second unfavourable step to occur. |
|||
| image_width = 230px |
|||
| regnum = [[Animal]]ia |
|||
=== Overall reaction catalysed === |
|||
| phylum = [[Chordate|Chordata]] |
|||
{{Enzymatic Reaction |
|||
| classis = [[Mammal]]ia |
|||
|foward_enzyme=[[glyceraldehyde phosphate dehydrogenase]] |
|||
| ordo = [[Carnivora]] |
|||
|reverse_enzyme= |
|||
| familia = [[Mustelidae]] |
|||
|substrate=[[glyceraldehyde 3-phosphate]] |
|||
| genus = '''''Gulo''''' |
|||
|product=<small>D</small>-[[glycerate 1,3-bisphosphate]] |
|||
| genus_authority = [[Peter Simon Pallas|Pallas]], 1780 |
|||
|reaction_direction_(forward/reversible/reverse)=reversible |
|||
| species = '''''G. gulo''''' |
|||
|minor_foward_substrate(s)=NAD<sup>+</sup> '''+''' P<sub>i</sub> |
|||
| binomial = ''Gulo gulo'' |
|||
|minor_foward_product(s)=NADH '''+''' H<sup>+</sup> |
|||
| binomial_authority = ([[Carolus Linnaeus|Linnaeus]], 1758) |
|||
|minor_reverse_substrate(s)=NADH '''+''' H<sup>+</sup> |
|||
| range_map = Leefgebied veelvraat.JPG |
|||
|minor_reverse_product(s)=NAD<sup>+</sup> '''+''' P<sub>i</sub> |
|||
| range_map_width = 200px |
|||
|substrate_image=D-glyceraldehyde-3-phosphate_wpmp.png |
|||
| range_map_caption = Wolverine range |
|||
|product_image=1,3-bisphospho-D-glycerate_wpmp.png |
|||
}} |
}} |
||
{{KEGG compound|C00118}} {{KEGG enzyme|1.2.1.12}} {{KEGG reaction|R01063}} {{KEGG compound|C00236}} |
|||
The '''wolverine''' (''Gulo gulo'') is the largest land-dwelling [[species]] of the [[Mustelidae]] or weasel family (the [[Giant Otter]] is largest overall) in the [[genus]] '''''Gulo''''' (meaning "glutton"). It is also called the '''Glutton''' or '''Carcajou'''. Some authors recognize two [[subspecies]]: the [[Old World]] form ''Gulo gulo gulo'' and the [[New World]] form ''G. g. luscus''. A third subspecies limited to [[Vancouver Island]] (''G. g. vancouverensis'') is also occasionally described; however, craniomorphic evidence suggests that the Vancouver Island wolverines are properly included within ''G. g. luscus''. |
|||
=== Two-step conversion of glyceraldehyde 3-phosphate=== |
|||
==Anatomy== |
|||
[[Anatomy|Anatomically]], the wolverine is a stocky and muscular animal. It has brown hair with stripes of dull yellow along the sides. Its fur is long and dense and does not retain much water, making it very resistant to frost, which is common in the wolverine's cold habitat. (For these reasons, the fur has been traditionally popular among hunters and trappers as a lining in jackets and parkas, especially for wear in Arctic conditions). The adult wolverine is about the size of a medium [[dog]], with a length usually ranging from 65-87 [[Centimetre|cm]] (25-34 [[inch]]es), a tail of 17-26 cm (7-10 inches), and weight of 9-17 [[Kilogram|kg]] (22-36 [[Pound (mass)|lb]]). The males are as much as 30 percent larger than the females. In appearance, the wolverine resembles a small bear with a long tail. It has been known to give off a very strong, extremely unpleasant odor, giving rise to the nicknames "skunk bear" and "nasty cat." Wolverines, as other mustelids, possess a special upper molar in the back of the mouth that is rotated 90 degrees, or sideways, towards the inside of the mouth. This special characteristic allows wolverines to tear off meat from prey or carrion that has been frozen solid and also to crush bones, which enables the wolverine to extract marrow.<ref>{{cite web | last = Pratt | first = Philip | title = Dentition of the Wolverine | publisher = The Wolverine Foundation, Inc. | url = http://www.wolverinefoundation.org/dentition.htm | accessdate = 2007-07-01}}</ref><ref name="akfishgame">{{cite web | last = Taylor | first = Ken | title = Wolverine | work = Wildlife Notebook Series | publisher = Alaska Department of Fish & Game | year = 1994 | url = http://www.adfg.state.ak.us/pubs/notebook/furbear/wolverin.php | format = HTML Public | accessdate = 2007-01-21}}</ref> |
|||
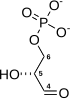
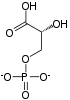
The first reaction is the oxidiation of [[glyceraldehyde 3-phosphate]] at the carbon 1 position (the 4th carbon from glycolysis which is shown in the diagram), in which an [[aldehyde]] is converted into a [[carboxylic acid]] (ΔG°'=-50 kJ/mol (-12kcal/mol)) and NAD+ is simultaneously reduced endergonically to NADH. The energy released by this highly [[exergonic]] oxidation reaction drives the [[endergonic]] second reaction (ΔG°'=+50 kJ/mol (+12kcal/mol)), in which a molecule of inorganic [[phosphate]] is transferred to the GAP intermediate to form a product with high phosphoryl-transfer potential: '''[[1,3-Biphosphoglycerate]]''' (1,3-BPG). This is an example of [[phosphorylation]] coupled to oxidation, and the overall reaction is somewhat endergonic (ΔG°'=+6.3 kJ/mol (+1.5)). Energy coupling here is made possible by GAPDH. |
|||
==Behavior== |
|||
[[Image:Gulo gulo-Woverine-Polar Zoo Norway.JPG|thumb|left|180px|Wolverine at the Polar Zoo, [[Bardu]] in Norway]] The wolverine is, like most mustelids, remarkably strong for its size. It has been known to kill prey as large as [[moose]], although most typically when these are weakened by winter or caught in snowbanks. Wolverines inhabiting the [[Old World]] (specifically, [[Fennoscandia]]) are more active hunters than their [[North America]]n cousins.<ref name="wwf">[http://www.wwf.se/source.php/1018447/Wolverine%20Symposium.pdf World Wildlife Fund–Sweden: 1st International Symposium on Wolverine Research and Management] (PDF)</ref> This may be because competing predator populations are not as dense, making it more practical for the wolverine to hunt for itself than to wait for another animal to make a kill and then try to snatch it. They often feed on [[carrion]] left by [[wolf|wolves]], so that changes in the population of wolves may affect the population of wolverines.<ref name="gr"/> Wolverines are also known on occasion to eat plant material.<ref name="rickert"/> |
|||
=== Mechanism of catalysis === |
|||
Armed with powerful jaws, sharp claws, and a thick hide,<ref name="biomes">[http://www.blueplanetbiomes.org/wolverine.htm World Biomes: Wolverine]</ref> wolverines may defend kills against larger or more numerous [[predators]].<ref name="youtube1">[http://youtube.com/watch?v=FlGnPlUJKRM&mode=related&search= YouTube: Wolverine challenges bear to leave] </ref> There is at least one published account of a 27-pound wolverine's attempt to steal a kill from a [[American Black Bear|black bear]] (adult males weigh 400 to 500 pounds). Unfortunately for the mustelid, the bear won what was ultimately a fatal contest.<ref name="WNS">{{cite press release | title = When Predators Attack (Each Other): Researchers Document First-known Killing Of A Wolverine By A Black Bear In Yellowstone| publisher = Science Daily | date = [[2003-05-06]] | url = http://www.sciencedaily.com/releases/2003/05/030506073236.htm | accessdate = 2007-01-16}}</ref> |
|||
GAPDH uses covalent catalysis and general base catalysis to decrease the very large and positive activation energy of the second step of this reaction. First, a [[cysteine]] residue in the active site of GAPDH attacks the carbonyl group of GAP, creating a [[hemithioacetal]] intermediate (covalent catalysis). Next, an adjacent, tightly bound molecule of [[NAD<sup>+</sup>]] accepts a [[hydride ion]] from GAP, forming [[NADH]]; GAP is concomitantly oxidized to a [[thioester]] intermediate using a molecule of water. This thioester species is much higher in energy than the [[carboxylic acid]] species that would result in the absence of GAPDH (the carboxylic acid species is so low in energy that the energy barrier for the second step of the reaction (phosphorylation) would be too great, and the reaction therefore too slow, for a living organism). Donation of the hydride ion by the hemithioacetal is facilitated by its deprotonation by a [[histidine]] residue in the enzyme's active site (general base catalysis). Deprotonation encourages the reformation of the carbonyl group in the thioester intermediate and ejection of the hydride ion. NADH leaves the active site and is replaced by another molecule of NAD<sup>+</sup>, the positive charge of which stabilizes the negatively-charged carbonyl oxygen in the transition state of the next and ultimate step. Finally, a molecule of [[inorganic phosphate]] attacks the thioester and forms a tetrahedral intermediate, which then collapses to release 1,3-bisphosphoglycerate, and the [[thiol]] group of the enzyme's cysteine residue. |
|||
Mating season is in the summer, but the actual implantation of the embryo (blastocyst) in the [[uterus]] is [[embryonic diapause|stayed]] until early winter, delaying the development of the [[fetus]]. Females will often not produce young if food is scarce. Litters of typically two or three young ("kits") are born in the spring. Kits develop rapidly, reaching adult size within the first year of a lifespan that may reach anywhere from five to (in exceptional individuals) thirteen years.{{Fact|date=March 2008}} |
|||
== Additional functions == |
|||
Adult wolverines have no natural predators, though they do come into conflict with (and may be killed by) other large predators over territory and food. Juveniles are of course more vulnerable; infants (kits) have been known on occasion to be taken by predatory birds such as eagles.<ref name="hinterland">[http://www.hww.ca/hww2.asp?id=108 Hinterland Who’s who: Wolverine]</ref> |
|||
GAPDH is multifunctional like an increasing number of enzymes. In addition to catalysing the 6th step of [[glycolysis]], recent evidence implicates GAPDH in other cellular processes. This came as a surprise to researchers but it makes evolutionary sense to re-use and adapt an existing proteins instead of evolving a novel protein from scratch. |
|||
==Range== |
|||
[[Image:Wolverine on rock.jpg|thumb|right|200px|Wolverine on rock]]The wolverine lives primarily in isolated northern areas, for example the [[arctic]] and [[alpine]] regions of [[Alaska]], northern [[Canada]], [[Siberia]] and [[Scandinavia]]; they are also native to [[Russia]] and the [[Baltic region|Baltic]] countries. The wolverine is arguably found as far south as the [[Sierra Nevada (U.S.)|Sierra Nevada]]<ref name="knudson">{{Citation|last=Knudson|first=Tom| title = Elusive wolverine makes its first Sierra appearance in years | newspaper = Sacramento Bee | year = 2008 | date = March 5, 2008 | url = http://www.sacbee.com/101/story/761071.html}}</ref> in [[California]], and a few remain in the [[Rocky Mountains]] and northern [[Cascades]] of the [[United States]]. However most of the Wolverines live in [[Canada]].<ref name="rickert">{{Citation|last=Rickert|first=Eve| title = The perils of secrecy | newspaper = High Country News | year = 2007 | date = June 28, 2007 | url = http://www.hcn.org/servlets/hcn.Article?article_id=17093}}</ref> |
|||
=== Transcription and apoptosis === |
|||
The world's total wolverine population is unknown. The animal exhibits a low population density and requires a very large home range.<ref name="gr">"[http://gristmill.grist.org/story/2008/3/4/12295/73009 Wolverine wonder]", [[Grist.org]], [[March 4]], [[2008]]; also {{cite web | url = http://www.cnn.com/2008/TECH/science/03/10/sierra.wolverine.ap/index.html | title = Student's camera snaps wolverine in California | date = 2008-03-10 | work = CNN.com | author = Associated Press | accessdate = 2008-03-11 }}</ref> The range of a male wolverine can be more than 620 [[Square kilometre|km²]] (240 [[Square miles|sq mi]]) while encompassing the ranges of several females (with smaller home ranges of roughly 130-260 km² (50-100 sq mi). Adult wolverines try for the most part to keep non-overlapping ranges with adults of the same sex.<ref name="akfishgame" /> Radio tracking suggests an animal can range hundreds of miles in only a few months. |
|||
Zheng et al. discovered in [[2003]] that GAPDH can itself activate [[transcription (genetics)|transcription]]. The ''OCA-S'' transcriptional coactivator complex contains GAPDH and [[lactate dehydrogenase]], two protein previously only thought to be involved in [[metabolism]]. GAPDH moves between the [[cytosol]] and the [[Cell nucleus|nucleus]] and may thus link the metabolic state to gene transcription. |
|||
{| class="wikitable" |
|||
<ref name="pmid12887926">{{cite journal |author=Zheng L, Roeder RG, Luo Y |title=S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component |journal=Cell |volume=114 |issue=2 |pages=255–66 |year=2003 |pmid=12887926 |doi=}}</ref> |
|||
|- |
|||
! Country |
|||
! Population |
|||
! Area |
|||
! Year |
|||
! State of Population |
|||
|- |
|||
| Sweden |
|||
| 265+<ref name="lcie-coe-ne-115">{{cite web |url=http://www.lcie.org/docs/COE/COE%20NE%20115%20Action%20plan%20for%20wolverines%202000.pdf |title=Action Plan for the conservation of Wolverines (Gulo gulo) in Europe |accessdate=2008-01-25 |author=Arild Landa, Mats Lindén and Ilpo Kojola |year=2000 |work=Nature and environment, No. 115 |publisher=Convention on the Conservation |
|||
of European Wildlife and Natural Habitats (Bern Convention) |
|||
|format=PDF}}</ref> |
|||
| [[Norrbotten]]<ref name="lcie-coe-ne-115"/> |
|||
| 1995-97<ref name="lcie-coe-ne-115"/> |
|||
| Stable<ref name="lcie-coe-ne-115"/> |
|||
|- |
|||
| Norway |
|||
| 150+<ref name="lcie-coe-ne-115"/> |
|||
| [[Snøhetta]] plateau and North<ref name="lcie-coe-ne-115"/> |
|||
| 1995-97<ref name="lcie-coe-ne-115"/> |
|||
| Decline<ref name="lcie-coe-ne-115"/> |
|||
|- |
|||
| Finland |
|||
| 115<ref name="lcie-coe-ne-115"/> |
|||
| [[Karelia]] and North<ref name="lcie-coe-ne-115"/> |
|||
| 1997<ref name="lcie-coe-ne-115"/> |
|||
| Stable<ref name="lcie-coe-ne-115"/> |
|||
|- |
|||
| Russia |
|||
| 1500<ref name="lcie-coe-ne-115"/> |
|||
| [[Taiga]]<ref name="lcie-coe-ne-115"/> |
|||
| 1970, 1990, <ref name="lcie-coe-ne-115"/> |
|||
| Decline<ref name="lcie-coe-ne-115"/> |
|||
|- |
|||
| Russia - [[Komi Republic|Komi]] |
|||
| 885<ref name="lcie-coe-ne-115"/> |
|||
| - |
|||
| 1990<ref name="lcie-coe-ne-115"/> |
|||
| - |
|||
|- |
|||
| Russia - [[Arkhangelsk Oblast|Archangelsk Oblast]] |
|||
| 410<ref name="lcie-coe-ne-115"/> |
|||
| [[Nenets Autonomous Okrug|Nenetsky Autonomous Area]]<ref name="lcie-coe-ne-115"/> |
|||
| 1990<ref name="lcie-coe-ne-115"/> |
|||
| Limited<ref name="lcie-coe-ne-115"/> |
|||
|- |
|||
| Russia - [[Kola Peninsula]] |
|||
| 160<ref name="lcie-coe-ne-115"/> |
|||
| Hunting Districts<ref name="lcie-coe-ne-115"/> |
|||
| 1990<ref name="lcie-coe-ne-115"/> |
|||
| Decline<ref name="lcie-coe-ne-115"/> |
|||
|- |
|||
| USA - [[Alaska]]<ref name="wf-kobuk">{{cite web |url=http://www.wolverinefoundation.org/research/kobuk.htm |title=population ecology of wolverines within Kobuk valley national park and Selawik national wildlife refuge |accessdate=2008-01-26 |author=Brad Shults, Gene Peltola, Jerrold Belant and Kyran Kunkel |date=12/17/98 |work=[[Rocky Mountain Research Station]], US Department of Agriculture - Forest Service |
|||
}}</ref> |
|||
| unknown<ref name="wf-kobuk"/> |
|||
| [[Kobuk Valley National Park]]<ref name="wf-kobuk"/>, [[Selawik National Wildlife Refuge]]<ref name="wf-kobuk"/> |
|||
| 1998<ref name="wf-kobuk"/> |
|||
| Decline<ref name="wf-kobuk"/> |
|||
|- |
|||
| USA - [[Alaska]]<ref name="bioone-wf-egw"> |
|||
{{cite web |
|||
|url=http://www.bioone.org/perlserv/?request=get-document&doi=10.2981%2F0909-6396(2007)13%5B52%3AEWGGPS%5D2.0.CO%3B2&ct=1 |
|||
|title=Estimating wolverine Gulo gulo population size using quadrat sampling of tracks in snow |
|||
|accessdate=2007 |
|||
|author=Howard N. Goldena, J. David Henryb, Earl F. Beckera, Michael I. Goldsteinc, John M. Mortond, Dennis Frost, and Aaron J. Poef |
|||
|date=12/17/98 |
|||
|work=Alaska Department of Fish and Game, Division of Wildlife Conservation; Parks Canada - Kluane National Park; US Forest Service - Alaska Regional Office; United States Fish and Wildlife Service, Kenai National Wildlife Refuge; North Yukon Renewable Resources Council; United States Forest Service, Chugach National Forest; |
|||
}}</ref> |
|||
| 3.0 (± 0.4 SE) wolverines/1,000 km<sup>2</sup><ref name="bioone-wf-egw"/> |
|||
| [[Cook Inlet|Turnagain Arm and the Kenai Mountains]]<ref name="bioone-wf-egw"/> |
|||
| 2004<ref name="bioone-wf-egw"/> |
|||
| -<ref name="bioone-wf-egw"/> |
|||
|- |
|||
| USA - [[California]]<ref name="gr"/> |
|||
| Unknown |
|||
| [[Tahoe National Forest]]<ref name="gr"/> |
|||
| 2008<ref name="gr"/> |
|||
| Unknown<ref name="gr"/> |
|||
|- |
|||
| Canada - [[Yukon]] |
|||
| 9.7 (± 0.6 SE) wolverines/1,000 km<sup>2</sup><ref name="bioone-wf-egw"/> |
|||
| [[Old Crow Flats]]<ref name="bioone-wf-egw"/> |
|||
| 2004<ref name="bioone-wf-egw"/> |
|||
| -<ref name="bioone-wf-egw"/> |
|||
|- |
|||
| Canada - [[Ontario]]<ref name="wf-ontario-pr">{{cite web |url=http://www.wolverinefoundation.org/research/Ontario%20Wolverine%20Project%20Report_July_04.pdf | title=Boreal Wolverine: A Focal Species for Land Use planning in Ontario's Northern Boreal Forest - Project Report |accessdate=2008-01-26 |author=Dr. Audrey Magoun, Neil Dawson, Dr. Geoff Lipsett-Moore, Dr. Justina C. Ray |year=2004 |work=The Wolverine Foundation, Inc., Ontario Ministry of Natural Resources, Ontario Parks, Wildlife Conservation Society (WCS)/University of Toronto |format=PDF}}</ref> |
|||
| unclear<ref name="wf-ontario-pr"/> |
|||
| [[Sioux Lookout|Red Lake – Sioux Lookout to Fort Severn]] – Peawanuck<ref name="wf-ontario-pr"/> |
|||
| 2004<ref name="wf-ontario-pr"/> |
|||
| Stable to Expanding<ref name="wf-ontario-pr"/> |
|||
|- |
|||
| Canada - Overall<ref name="gg-sr"> |
|||
{{cite web |
|||
|url=http://dsp-psd.pwgsc.gc.ca/Collection/CW69-14-329-2003E.pdf |
|||
|title=COSEWIC Assessment and Update Status Report on the Wolverine (Gulo gulo) - Eastern Population Western Population in Canada |
|||
|accessdate=2008-01-26 |
|||
|author=Brian Slough et al. |
|||
|month=May | year=2003 |
|||
|work=COSEWIC (committee on the status of endangered wildlife in Canada) 2003. COSEWIC assessment and update status report on the wolverine Gulo gulo in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 41 pp. |
|||
|format=PDF}}</ref> |
|||
| 15000 to 19000<ref name="gg-sr"/> |
|||
| Overall<ref name="gg-sr"/> |
|||
| -<ref name="gg-sr"/> |
|||
| Stable<ref name="gg-sr"/> |
|||
|} |
|||
In [[2005]], Hara et al. showed that GAPDH initiates [[apoptosis]]. This is not a third function, but can be seen as an activity mediated by GAPDH binding to [[DNA]] like in transcription activation, discussed above. The study demonstrated that GAPDH is [[Nitric oxide#Biological_functions|S-nitrosylated]] by NO in response to cell stress, which causes it to bind to the protein ''Siah1'', a [[ubiquitin ligase]]. The complex moves into the nucleus where Siah1 targets nuclear proteins for [[protein degradation|degradation]], thus initiating controlled cell shutdown. |
|||
This requirement for large territories brings wolverines into conflict with human development, and hunting and trapping further reduce their numbers, causing them to disappear from large parts of their former range; attempts to have them declared an endangered species have met with little success.<ref name="gr"/> |
|||
<ref name="pmid15951807">{{cite journal |author=Hara MR, Agrawal N, Kim SF, ''et al'' |title=S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding |journal=Nat. Cell Biol. |volume=7 |issue=7 |pages=665–74 |year=2005 |pmid=15951807 |doi=10.1038/ncb1268}}</ref> |
|||
In subsequent study the group demonstrated that [[deprenyl]], which has been used clinically to treat [[Parkinson's disease]], strongly reduces the apoptotic action of GAPDH by preventing its S-nitrosylation and might thus be used as a drug. |
|||
<ref name="pmid16505364">{{cite journal |author=Hara MR, Thomas B, Cascio MB, ''et al'' |title=Neuroprotection by pharmacologic blockade of the GAPDH death cascade |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=103 |issue=10 |pages=3887–9 |year=2006 |pmid=16505364 |doi=10.1073/pnas.0511321103}}</ref> |
|||
== Metabolic Switch == |
|||
==Name== |
|||
The wolverine's (questionable) reputation as an insatiable glutton may be in part due to a [[false etymology]]. The animal's name in old [[Swedish language|Swedish]], ''Fjellfräs'', meaning "[[fell]] (mountain) cat", worked its way into German as ''Vielfraß'', which means roughly "devours much". Its name in other West Germanic languages is similar (e.g. Dutch ''Veelvraat''). The name in [[Old Norse]], ''Jarfr'', lives on in the regular [[Icelandic language|Icelandic]] name ''jarfi'', regular [[Norwegian language|Norwegian]] name ''jerv'', regular Swedish name ''järv'' and regular [[Danish language|Danish]] name ''jærv''. The Finnish name is Ahma, which is derived from "ahmia" which also is roughly translated as "devours much". |
|||
GAPDH acts as reversible metabolic switch under oxidative stress. When cells are exposed to [[oxidant]]s, they need excessive amounts of the antioxidant cofactor [[NADPH]]. In the cytosol, NADPH is reduced from NADP+ by several enzymes, three of them catalyze the first steps of the [[Pentose Phosphate Pathway]]. Oxidant-treatments cause an inactivation of GAPDH. This inactivation re-routes temporally the metabolic flux from glycolysis to the Pentose Phosphate Pathway, allowing the cell to generate more NADPH. <ref name="pmid18154684">{{cite journal |author=Ralser M et al |title=Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. |journal=J Biol |volume=6 |issue=11 |year=2007 |pmid=18154684}}</ref>. Under stress conditions, NADPH is needed by some antioxidant-systems including [[glutaredoxin]] and [[thioredoxin]] as well as being essential for the recycling of [[gluthathione]]. |
|||
==As a symbol== |
|||
=== ER to Golgi transport === |
|||
The Norwegian municipality of [[Bardu]] and Finnish municipality of [[Kittilä]] have a wolverine in their coats-of-arms. |
|||
GAPDH also appears to be involved in the [[Vesicle (biology)#Vesicle_formation_and_transport|vesicle transport]] from the [[endoplasmic reticulum]] (ER) to the [[Golgi apparatus]] which is part of shipping route for secreted proteins. It was found that GAPDH is recruited by [[Rab (G-protein)|rab2]] to the [[vesicular-tubular clusters]] of the ER where it helps to form [[COPI|COP 1 vesicles]]. GAPDH is activated via [[tyrosine]] [[phosphorylation]] by [[Src (gene)|Src]]. |
|||
The [[U.S. state]] of [[Michigan]] is, by tradition, known as "The Wolverine State," and the [[University of Michigan]] takes the wolverine as its mascot. Many other educational institutions utilized the wolverine as an athletic mascot (e.g., [[Bronx High School of Science]] and [[Utah Valley University]]). A major league baseball team from the 1880s was also popularly known as the "[[Detroit Wolverines]]". The association is well and long established: for example, many [[Detroit]]ers volunteered to fight during the [[American Civil War]] and [[George Armstrong Custer]], who led the Michigan Brigade, called them the "Wolverines." The origins of this association are obscure: it may derive from a busy trade in wolverine furs in [[Sault Ste. Marie, Michigan|Sault Ste. Marie]] in the 18th century or may recall a disparagement intended to compare early settlers in Michigan with the vicious mammal. In any event, the animal appears no longer to be indigenous to the state (and in fact may never have been). It is, at the very least, an uncommon sight there: for example, when one was observed in February 2004<ref name="msnbc">[http://www.msnbc.msn.com/id/4374309/ MSNBC: First Michigan wolverine spotted in 200 years]</ref> by [[hunting|hunters]] and [[biologist]]s near [[Ubly, Michigan|Ubly]], it was the first confirmed sighting of a wolverine in Michigan in about two centuries. It is unknown whether that particular animal was a state native or if it migrated or had been released by humans. |
|||
<ref name="pmid17488287">{{cite journal |author=Tisdale EJ, Artalejo CR |title=A GAPDH mutant defective in Src-dependent tyrosine phosphorylation impedes Rab2-mediated events |journal=Traffic |volume=8 |issue=6 |pages=733–41 |year=2007 |pmid=17488287 |doi=10.1111/j.1600-0854.2007.00569.x}}</ref> |
|||
== Cellular location == |
|||
The [[European Football League]] (playing [[American football]] in [[Europe]]) includes the Helsinki Wolverines,<ref name="helsinki">[http://wolverines.dt-link.fi Helsinki Wolverines]</ref> founded in 1995. The team plays in the Maple League, the Finnish top level. |
|||
The wolverine figures prominently in the mythology of the [[Innu]] people of eastern [[Québec]] and [[Labrador]]. In at least one Innu myth, it is the creator of the world.<ref name="Armitage">{{cite journal | last = Armitage| first = Peter | title = Religious ideology among the Innu of eastern Quebec and Labrador | journal = Religiologiques | volume = 6 | year = 1992 | url = http://www.er.uqam.ca/nobel/religio/no6/armit.pdf | accessdate = 2007-06-29|format=PDF}} (PDF)</ref> |
|||
All steps of glycolysis take place in the [[cytosol]] and so does the reaction catalysed by GAPDH. Research in [[red blood cells]] indicates that GAPDH and several other glycolytic enzymes assemble in complexes on the inside of the [[cell membrane]]. The process appears to be regulated by phosphorylation and oxygenation. |
|||
In the Central Interior Hockey League, founded in 1996,<ref name="CIHL">[http://www.cihl.info/ CIHL]</ref> in [[British Columbia]], [[Canada]], an [[ice hockey]] team team based in [[Hazelton, British Columbia]] is named the Hazelton Wolverines.<ref name="Hazelton">[http://www.hazeltonwolverines.com/ Hazelton Wolverines]</ref> |
|||
<ref name="pmid15701694">{{cite journal |author=Campanella ME, Chu H, Low PS |title=Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=102 |issue=7 |pages=2402–7 |year=2005 |pmid=15701694 |doi=10.1073/pnas.0409741102}}</ref> |
|||
Bringing several glycolytic enzymes close to each other is expected to greatly increased the overall speed of glucose breakdown. |
|||
== Miscellaneous == |
|||
Because the GAPDH gene is often stably and constitutively expressed at high levels in most tissues and cells, it is considered a [[housekeeping gene]]. For this reason, GAPDH is commonly used by biological researchers as a loading control for [[western blot]] and as a control for [[RT-PCR]]. However, many researchers report different regulation of GAPDH under specific conditions. Therefore, the use of GAPDH as loading control has to be controlled carefully. |
|||
<!-- Please do not add your high school's mascot here. These are not notable and the mention will be removed. --> |
|||
== |
== Sources == |
||
=== Glycolysis text book references === |
|||
{{wikispecies|Gulo gulo}} |
|||
*Voet, D. and Voet, J. G. (2004) ''Biochemistry'', Third Edition. J. Wiley & Sons, Hoboken, NJ. |
|||
{{commons|Gulo gulo}} |
|||
*Berg, Jeremy M., Tymoczko, John L., & Stryer, Lubert (2007) ''Biochemistry'', Sixth Edition. W. H. Freeman and Co., NY. |
|||
*[http://www.ncbi.nlm.nih.gov/books/bv.fcgi?highlight=glyceraldehyde+3+phosphate+dehydrogenase&rid=mcb.figgrp.4342&WebEnv=0bpB8XePphZ8qSS3b9o1BB3FMZtXPr7yFc3MxfLR12WUi7sKapf987mBijj9A0v-LwF_W_lLjUKNwY%40D45D6EC76612AEB0_0018SID&WebEnvRq=1 diagram of the GAPDH reaction mechanism] from Lodish MCB at NCBI bookshelf |
|||
*[http://www.ncbi.nlm.nih.gov/books/bv.fcgi?highlight=glyceraldehyde+3+phosphate+dehydrogenase&rid=mboc4.figgrp.297&WebEnv=0qL7ctlqrxJxTMzSHUlui3y2aeU6B8K6Tblugar02bi5Eetekc7g1j_m9gRDhWr1NM3L7U4G-5GFjf%40D45D6EC76612AEB0_0018SID&WebEnvRq=1 similar diagram] from Alberts The Cell at NCBI bookshelf |
|||
=== Cited research === |
|||
{{reflist}} |
{{reflist}} |
||
---- |
|||
==External links== |
|||
* [http://www.lcie.org/res_sps_wolverine.htm Large Carnivore Initiative for Europe: Wolverine]: scientific articles about wolverines |
|||
{{glycolysis}} |
|||
<!-- This could probably be used as source, but not as external link: [http://timberjay.com/current.php?article=4275 March 2008 sighting near Ely, Mn]--> |
|||
{{Glycolysis enzymes}} |
|||
{{Aldehyde/Oxo oxidoreductases}} |
|||
<!-- The PBB_Controls template provides controls for Protein Box Bot, please see Template:PBB_Controls for details. --> |
|||
== Gallery== |
|||
{{PBB_Controls |
|||
<gallery> |
|||
| update_page = yes |
|||
Image:Brehms Het Leven der Dieren Zoogdieren Orde 4 Veelvraat (Gulo borealis).jpg |
|||
| require_manual_inspection = no |
|||
Image:Wolverine display at Arctic Interagency Visitor Center at Coldfoot.jpg |
|||
| update_protein_box = yes |
|||
</gallery> |
|||
| update_summary = no |
|||
| update_citations = no |
|||
{{Mustelidae nav}} |
|||
}} |
|||
{{Red List Canada}} |
|||
[[Category: |
[[Category:EC 1.2.1]] |
||
[[Category:Mammals of Canada]] |
|||
[[Category:Mammals of North America]] |
|||
[[Category:Mammals of Europe]] |
|||
[[Category:Mammals of Asia]] |
|||
[[Category:Arctic land animals]] |
|||
[[Category:Wildlife of the Arctic]] |
|||
[[Category:Fauna of Finland]] |
|||
[[bg:Глицералдехид-3-фосфатдехидрогеназа]] |
|||
[[br:Karkajou]] |
|||
[[de:Glycerinaldehyd-3-phosphat-Dehydrogenase]] |
|||
[[bg:Росомаха]] |
|||
[[es:Gliceraldehído-3-fosfato deshidrogenasa]] |
|||
[[cs:Rosomák sibiřský]] |
|||
[[da:Jærv]] |
|||
[[de:Vielfraß]] |
|||
[[et:Ahm]] |
|||
[[es:Gulo gulo]] |
|||
[[eo:Gulo]] |
|||
[[fr:Glouton]] |
|||
[[hr:Gorska kuna]] |
|||
[[is:Jarfi]] |
|||
[[it:Gulo gulo]] |
|||
[[he:גרגרן]] |
|||
[[lt:Ernis]] |
|||
[[hu:Rozsomák]] |
|||
[[nl:Veelvraat (zoogdier)]] |
|||
[[ja:クズリ]] |
|||
[[no:Jerv]] |
|||
[[nn:Jerv]] |
|||
[[pl:Rosomak]] |
|||
[[pt:Glutão]] |
|||
[[ru:Росомаха]] |
|||
[[sah:Сиэгэн]] |
|||
[[simple:Wolverine]] |
|||
[[fi:Ahma]] |
|||
[[sv:Järv]] |
|||
[[vi:Chồn gulô]] |
|||
[[uk:Росомаха]] |
|||
[[zh:貂熊]] |
|||
Revision as of 02:59, 12 October 2008
Glyceraldehyde 3-phosphate dehydrogenase (abbreviated as GAPDH or less commonly as G3PDH) (EC 1.2.1.12) is an enzyme that catalyzes the sixth step of glycolysis and thus serves to break down glucose for energy and carbon molecules. In addition to this long established metabolic function, GAPDH has recently been implicated in several non-metabolic processes, including transcription activation, initiation of apoptosis [1] , and ER to Golgi vesicle shuttling.
Metabolic function
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) catalyses the conversion of glyceraldehyde 3-phosphate as the name indicates. This is the 6th step of the breakdown of glucose (glycolysis), an important pathway of energy and carbon molecule supply located in the cytosol of eukaryotic cells. Glyceraldehyde 3-phosphate is converted to D-glycerate 1,3-bisphosphate in two coupled steps. The first is favourable and allows the second unfavourable step to occur.
Overall reaction catalysed
| glyceraldehyde 3-phosphate | {{{forward_enzyme}}} | D-glycerate 1,3-bisphosphate | |

|

| ||
| {{{minor_forward_substrate(s)}}} | {{{minor_forward_product(s)}}} | ||

| |||
| NAD+ + Pi | NADH + H+ | ||
Compound C00118 at KEGG Pathway Database. Enzyme 1.2.1.12 at KEGG Pathway Database. Reaction R01063 at KEGG Pathway Database. Compound C00236 at KEGG Pathway Database.
Two-step conversion of glyceraldehyde 3-phosphate
The first reaction is the oxidiation of glyceraldehyde 3-phosphate at the carbon 1 position (the 4th carbon from glycolysis which is shown in the diagram), in which an aldehyde is converted into a carboxylic acid (ΔG°'=-50 kJ/mol (-12kcal/mol)) and NAD+ is simultaneously reduced endergonically to NADH. The energy released by this highly exergonic oxidation reaction drives the endergonic second reaction (ΔG°'=+50 kJ/mol (+12kcal/mol)), in which a molecule of inorganic phosphate is transferred to the GAP intermediate to form a product with high phosphoryl-transfer potential: 1,3-Biphosphoglycerate (1,3-BPG). This is an example of phosphorylation coupled to oxidation, and the overall reaction is somewhat endergonic (ΔG°'=+6.3 kJ/mol (+1.5)). Energy coupling here is made possible by GAPDH.
Mechanism of catalysis
GAPDH uses covalent catalysis and general base catalysis to decrease the very large and positive activation energy of the second step of this reaction. First, a cysteine residue in the active site of GAPDH attacks the carbonyl group of GAP, creating a hemithioacetal intermediate (covalent catalysis). Next, an adjacent, tightly bound molecule of [[NAD+]] accepts a hydride ion from GAP, forming NADH; GAP is concomitantly oxidized to a thioester intermediate using a molecule of water. This thioester species is much higher in energy than the carboxylic acid species that would result in the absence of GAPDH (the carboxylic acid species is so low in energy that the energy barrier for the second step of the reaction (phosphorylation) would be too great, and the reaction therefore too slow, for a living organism). Donation of the hydride ion by the hemithioacetal is facilitated by its deprotonation by a histidine residue in the enzyme's active site (general base catalysis). Deprotonation encourages the reformation of the carbonyl group in the thioester intermediate and ejection of the hydride ion. NADH leaves the active site and is replaced by another molecule of NAD+, the positive charge of which stabilizes the negatively-charged carbonyl oxygen in the transition state of the next and ultimate step. Finally, a molecule of inorganic phosphate attacks the thioester and forms a tetrahedral intermediate, which then collapses to release 1,3-bisphosphoglycerate, and the thiol group of the enzyme's cysteine residue.
Additional functions
GAPDH is multifunctional like an increasing number of enzymes. In addition to catalysing the 6th step of glycolysis, recent evidence implicates GAPDH in other cellular processes. This came as a surprise to researchers but it makes evolutionary sense to re-use and adapt an existing proteins instead of evolving a novel protein from scratch.
Transcription and apoptosis
Zheng et al. discovered in 2003 that GAPDH can itself activate transcription. The OCA-S transcriptional coactivator complex contains GAPDH and lactate dehydrogenase, two protein previously only thought to be involved in metabolism. GAPDH moves between the cytosol and the nucleus and may thus link the metabolic state to gene transcription. [2]
In 2005, Hara et al. showed that GAPDH initiates apoptosis. This is not a third function, but can be seen as an activity mediated by GAPDH binding to DNA like in transcription activation, discussed above. The study demonstrated that GAPDH is S-nitrosylated by NO in response to cell stress, which causes it to bind to the protein Siah1, a ubiquitin ligase. The complex moves into the nucleus where Siah1 targets nuclear proteins for degradation, thus initiating controlled cell shutdown. [3] In subsequent study the group demonstrated that deprenyl, which has been used clinically to treat Parkinson's disease, strongly reduces the apoptotic action of GAPDH by preventing its S-nitrosylation and might thus be used as a drug. [4]
Metabolic Switch
GAPDH acts as reversible metabolic switch under oxidative stress. When cells are exposed to oxidants, they need excessive amounts of the antioxidant cofactor NADPH. In the cytosol, NADPH is reduced from NADP+ by several enzymes, three of them catalyze the first steps of the Pentose Phosphate Pathway. Oxidant-treatments cause an inactivation of GAPDH. This inactivation re-routes temporally the metabolic flux from glycolysis to the Pentose Phosphate Pathway, allowing the cell to generate more NADPH. [5]. Under stress conditions, NADPH is needed by some antioxidant-systems including glutaredoxin and thioredoxin as well as being essential for the recycling of gluthathione.
ER to Golgi transport
GAPDH also appears to be involved in the vesicle transport from the endoplasmic reticulum (ER) to the Golgi apparatus which is part of shipping route for secreted proteins. It was found that GAPDH is recruited by rab2 to the vesicular-tubular clusters of the ER where it helps to form COP 1 vesicles. GAPDH is activated via tyrosine phosphorylation by Src. [6]
Cellular location
All steps of glycolysis take place in the cytosol and so does the reaction catalysed by GAPDH. Research in red blood cells indicates that GAPDH and several other glycolytic enzymes assemble in complexes on the inside of the cell membrane. The process appears to be regulated by phosphorylation and oxygenation. [7] Bringing several glycolytic enzymes close to each other is expected to greatly increased the overall speed of glucose breakdown.
Miscellaneous
Because the GAPDH gene is often stably and constitutively expressed at high levels in most tissues and cells, it is considered a housekeeping gene. For this reason, GAPDH is commonly used by biological researchers as a loading control for western blot and as a control for RT-PCR. However, many researchers report different regulation of GAPDH under specific conditions. Therefore, the use of GAPDH as loading control has to be controlled carefully.
Sources
Glycolysis text book references
- Voet, D. and Voet, J. G. (2004) Biochemistry, Third Edition. J. Wiley & Sons, Hoboken, NJ.
- Berg, Jeremy M., Tymoczko, John L., & Stryer, Lubert (2007) Biochemistry, Sixth Edition. W. H. Freeman and Co., NY.
- diagram of the GAPDH reaction mechanism from Lodish MCB at NCBI bookshelf
- similar diagram from Alberts The Cell at NCBI bookshelf
Cited research
- ^ A. Tarze, A. Deniaud, M. Le Bras, E. Maillier, D. Molle, N. Larochette, N. Zamzami, G. Jan, G. Kroemer, and C. Brenner (2007). "GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization". Oncogene. 26 (18): 2606–2620. doi:10.1038/sj.onc.1210074. PMID 17072346.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zheng L, Roeder RG, Luo Y (2003). "S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component". Cell. 114 (2): 255–66. PMID 12887926.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hara MR, Agrawal N, Kim SF; et al. (2005). "S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding". Nat. Cell Biol. 7 (7): 665–74. doi:10.1038/ncb1268. PMID 15951807.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Hara MR, Thomas B, Cascio MB; et al. (2006). "Neuroprotection by pharmacologic blockade of the GAPDH death cascade". Proc. Natl. Acad. Sci. U.S.A. 103 (10): 3887–9. doi:10.1073/pnas.0511321103. PMID 16505364.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Ralser M; et al. (2007). "Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress". J Biol. 6 (11). PMID 18154684.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Tisdale EJ, Artalejo CR (2007). "A GAPDH mutant defective in Src-dependent tyrosine phosphorylation impedes Rab2-mediated events". Traffic. 8 (6): 733–41. doi:10.1111/j.1600-0854.2007.00569.x. PMID 17488287.
- ^ Campanella ME, Chu H, Low PS (2005). "Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane". Proc. Natl. Acad. Sci. U.S.A. 102 (7): 2402–7. doi:10.1073/pnas.0409741102. PMID 15701694.
{{cite journal}}: CS1 maint: multiple names: authors list (link)