Enanthotoxin
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
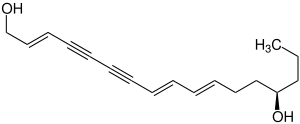
|
||||||||||
| General | ||||||||||
| Surname | Enanthotoxin | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 17 H 22 O 2 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 258.36 g mol −1 | |||||||||
| Melting point |
87 ° C |
|||||||||
| solubility |
soluble in ethanol , diethyl ether and chloroform |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Oenanthotoxin is a chemical compound from the group of alkynols and picrotoxins. It is structurally similar to Cicutoxin and was first isolated by Rudolf Boehm in 1876 and analyzed by EFLJ Anet in 1953.
Occurrence
Oenanthotoxin occurs naturally in the saffron vine umbel ( Oenanthe crocata ). The compound is poisonous and causes poisoning in horses and cows.
Extraction and presentation
Ekkehard Winterfeldt was primarily concerned with the synthesis of enanthotoxin .
Individual evidence
- ↑ a b N. Barsel, AJ Birch, F. Bohlmann , H. Brockmann, H. Brown, JD Chanley, HJ Mannhardt, RA Morton, GAJ Pitt, H. Sobotka, C. Tamm: Progress in the chemistry of organic natural substances / Progress in the Chemistry of Organic Natural Products / Progrès Dans la Chimie des Substances Organiques Naturelles . Springer-Verlag, 2013, ISBN 978-3-7091-7164-6 , pp. 1 ( limited preview in Google Book search).
- ^ Annual report of the pharmacy . Deutscher Apotheker-Verlag, 1895, p. 211 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Donald G. Barceloux: Medical Toxicology of Natural Substances Foods, Fungi, Medicinal Herbs, Plants, and Venomous Animals . John Wiley & Sons, 2008, ISBN 978-0-471-72761-3 , pp. 822 ( limited preview in Google Book search).
- ↑ Sven Sommerwerk, Lucie Heller, Bianka Siewert, René Csuk: Chemoenzymatic synthesis and cytotoxicity of oenanthotoxin and analogues. In: Bioorganic & Medicinal Chemistry. 23, 2015, p. 5595, doi: 10.1016 / j.bmc.2015.07.031 .
- ↑ Else Petri: Pathological anatomy and histology of poisonings . Springer-Verlag, 2013, ISBN 978-3-7091-6003-9 , pp. 446 ( limited preview in Google Book search).
- ↑ Mark Kalesse: Ekkehard Winterfeldt (1932-2014). In: Angewandte Chemie. 127, 2015, p. 35, doi: 10.1002 / anie.201410838 .