α-isomethylionone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
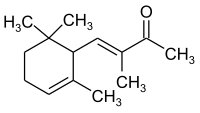
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Α-isomethylionone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 14 H 22 O | ||||||||||||||||||
| Brief description |
light yellow liquid with a floral odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 206.32 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.93 g cm −3 (20 ° C) |
||||||||||||||||||
| boiling point |
93 ° C (3.1 mmHg) |
||||||||||||||||||
| Refractive index |
1.5000-1.5020 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
α-Isomethylionon is a chemical compound from the group of cycloalkenes . The compound is one of the isomers of methylionone .
Extraction and presentation
α-Isomethylionone can be produced by a crossed aldol condensation of citral with methyl ethyl ketone , with the 7,11- as an intermediate stage with the desired methyl pseudoionone (3,6,10-trimethyl-3,5,9-undecatrien-2-one) as a by-product Dimethyl-4,6,10-dodecatrien-3-one is formed. The methyl pseudoionone is then cyclized to a mixture of α- and β-isomethylionone using a strong alkali compound and high temperature .
properties
α-Isomethylionone is a light yellow liquid with a floral odor.
use
α-Isomethylionone is a fragrance that is often added to deodorants and perfumes. It is one of the allergens that are responsible for axillary dermatitis . The compound is used for studies on contact sensitization to identify allergens in cosmetic preparations.
Individual evidence
- ↑ a b c d e f g data sheet α-Isomethylionone, odorant used in allergy studies at Sigma-Aldrich , accessed on December 8, 2018 ( PDF ).
- ↑ a b Wolfgang Legrum: Fragrances, between stench and fragrance Occurrence, properties and use of fragrances and their mixtures . Springer-Verlag, 2011, ISBN 978-3-8348-8276-9 , pp. 143 ( limited preview in Google Book search).
- ↑ a b c George A. Burdock: Encyclopedia of Food & Color Additives . CRC Press, 2014, ISBN 978-1-4987-1108-1 , pp. 1476 ( limited preview in Google Book search).

