Divinylbenzenes
| Divinylbenzenes | ||||||||
| Surname | o -divinylbenzene | m -divinylbenzene | p -divinylbenzene | |||||
| other names | 1,2-divinylbenzene, 1,2-diethenylbenzene |
1,3-divinylbenzene, 1,3-diethenylbenzene |
1,4-divinylbenzene, 1,4-diethenylbenzene |
|||||
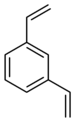
| Structural formula |

|
|

|
|||||
| CAS number | 91-14-5 | 108-57-6 | 105-06-6 | |||||
| 1321-74-0 (mixture of isomers) | ||||||||
| PubChem | 66666 | 7941 | 66041 | |||||
| Molecular formula | C 10 H 10 | |||||||
| Molar mass | 130.19 g mol −1 | |||||||
| Physical state | liquid | firmly | ||||||
| Brief description | water-clear, irritating to the mucous membranes, easily polymerizable, flammable liquid |
combustible solid | ||||||
| Melting point | −67 ° C | −67 ° C | 31 ° C | |||||
| boiling point | 200 ° C | 200 ° C | 95–96 ° C (24 hPa ) | |||||
| solubility | - | 52 mg / l (25 ° C) | practically insoluble in water | |||||
|
GHS labeling |
|
|
|
|||||
| H and P phrases | 315-319-335-411 | see above | 315-319-335-411 | |||||
| no EUH phrases | see above | no EUH phrases | ||||||
|
273-302 + 352-304 + 340 305 + 351 + 338 |
see above |
273-302 + 352-304 + 340 305 + 351 + 338 |
||||||
The divinylbenzenes are aromatic hydrocarbons having the general formula C 10 H 10 . They consist of a benzene ring with two vinyl groups (–CH = CH 2 ) as substituents . Their different arrangement ( ortho , meta or para ) result in three constitutional isomers . Technical divinylbenzene is a mixture of the ortho , meta and para isomers (1,2-, 1,3- and 1,4-divinylbenzene).
properties
o - and m -Divinylbenzene are water-clear, mucous membrane-irritating, easily polymerizable, flammable liquids. The p -divinylbenzene, which has the highest symmetry, has the highest melting point and is a solid. They are insoluble in water.
use
Divinylbenzenes are mainly used as crosslinkers in the production of styrene copolymers , which results in reduced solubility in most organic solvents , improved heat resistance, higher hardness and strength without affecting the appearance or optical and electrical properties. By far the largest amount is consumed in the production of copolymers with styrene derivatives for use as ion exchange material . As a rule, technical mixtures are polymerized with a proportion of 55% or 80%.
Individual evidence
- ↑ Entry for CAS no. 1321-74-0 in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ↑ a b c d Entry on 1,2-divinylbenzene in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ↑ a b c Entry on 1,3-divinylbenzene in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .
- ↑ a b c d Entry on 1,4-divinylbenzene in the GESTIS substance database of the IFA , accessed on March 13, 2017(JavaScript required) .

