2,3-dimethyl-2-butene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
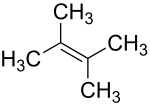
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,3-dimethyl-2-butene | |||||||||||||||
| other names |
Tetramethylethylene |
|||||||||||||||
| Molecular formula | C 6 H 12 | |||||||||||||||
| Brief description |
colorless liquid with a mild odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 84.16 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.71 g cm −3 |
|||||||||||||||
| Melting point |
−74 ° C |
|||||||||||||||
| boiling point |
73 ° C |
|||||||||||||||
| Vapor pressure |
133 hPa (20 ° C) |
|||||||||||||||
| solubility |
practically insoluble in water (0.071 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.412 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2,3-Dimethyl-2-butene is a chemical compound from the group of aliphatic , unsaturated hydrocarbons .
Extraction and presentation
2,3-dimethyl-2-butene can be prepared by dehydrogenation of 3,3-dimethyl-2-butanol can be obtained.
properties
2,3-Dimethyl-2-butene is a highly flammable, volatile, colorless liquid with a mild odor, which is practically insoluble in water.
use
2,3-Dimethyl-2-butene is used as an intermediate in the manufacture of other chemical compounds (e.g. pharmaceuticals). The ozonolysis of pure 2,3-dimethyl-2-butene yields Tetramethylenepoxid as a product.
safety instructions
The vapors of 2,3-dimethyl-2-butene can form an explosive mixture with air ( flash point <−20 ° C, ignition temperature 400 ° C).
Individual evidence
- ↑ a b c d e f g h i j k Entry on 2,3-dimethyl-2-butene in the GESTIS substance database of the IFA , accessed on July 11, 2018(JavaScript required) .
- ↑ Data sheet 2,3-dimethyl-2-butene, ≥99% from Sigma-Aldrich , accessed on July 11, 2018 ( PDF ).
- ^ Richard OC Norman, James M. Coxon: Principles of Organic Synthesis, 3rd Edition . CRC Press, 1993, ISBN 978-0-7487-6162-3 , pp. 456 ( limited preview in Google Book search).
- ↑ William H. Brown, Christopher S. Foote, Brent L. Iverson, Eric Anslyn: Organic Chemistry . Cengage Learning, 2011, ISBN 978-0-8400-5498-2 ( limited preview in Google Book Search).
- ↑ Data sheet Tetramethylethylene, 97% at AlfaAesar, accessed on July 11, 2018 ( PDF )(JavaScript required) .
- ^ Robert W. Murray, Wei Kong, Shirish N. Rajadhyaksha: The ozonolysis of tetramethylethylene. Concentration and temperature effects. In: The Journal of Organic Chemistry. 58, 1993, p. 315, doi : 10.1021 / jo00054a010 .

