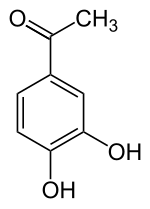
3,4-dihydroxyacetophenone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3,4-dihydroxyacetophenone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 8 O 3 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 152.15 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
116 ° C |
|||||||||||||||
| boiling point |
127-133 ° C (11 torr) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
3,4-dihydroxyacetophenone ( acetoprotocatechone , 4-acetylcatechol ) is an aromatic compound that is derived from both acetophenone and catechol (1,2-dihydroxybenzene). The structure consists of a benzene ring with an attached acetyl group (-COCH 3 ) and two hydroxyl groups (-OH) as substituents . It has anti-inflammatory properties.
In Erich Neitzel's work, the analogy of name choice for the derivatives of acetovanillion is used, e.g. E.g .: Acetoprotocatechon , derived from Protocatechu aldehyde , and Acetoveratrone ( 3,4-dimethoxyacetophenone , derived from Veratrum aldehyde ).
presentation
The representation is possible, for example, by demethylation of acetovanillone with dilute hydrochloric acid at 140-150 ° C in a closed vessel.
Web links
- Entry to 3,4-dihydroxyacetophenones . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed November 17, 2012.
Individual evidence
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 , p. 177, no. 148.
- ↑ a b Entry on 3 ′, 4′-Dihydroxyacetophenone at TCI Europe, accessed on November 1, 2016.
- ↑ You Jung Kim, Jae Kyung No, Jun Sik Lee, Myoung Soo Kim, Hae Young Chung: Antimelanogenic Activity of 3,4-Dihydroxyacetophenone: Inhibition of Tyrosinase and MITF , in: Bioscience, Biotechnology, and Biochemistry , 2006 , 70 (2 ), Pp. 532-534; doi: 10.1271 / bbb.70.532 ; PMID 16495675 ; PDF .
- ↑ Ping Wu, Li Zhang, Xiaoyan Zhou, Yongsheng Li, Daijuan Zhang, Jinyuan Wan, Duyun Ye: Inflammation pro-resolving potential of 3,4-dihydroxyacetophenone through 15-deoxy-Δ 12,14 -prostaglandin J 2 in murine macrophages , in: International Immunopharmacology , 2007 , 7 (11), pp. 1450-1459; doi: 10.1016 / j.intimp.2007.06.008 ; PMID 17761349 .
- ↑ Erich Neitzel: Das Acetovanillon und seine Derivate (Diss.), Berlin 1890, pressure from. Thormann & Goetsch, 40 pages + 2 pages
- ↑ a b Erich Neitzel: Ueber Derivate des Acetovanillons , in: Reports of the German Chemical Society , 1891 , 24 (2), pp. 2863-2868; doi: 10.1002 / cber.189102402111 .
