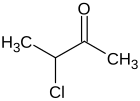
3-chlorobutan-2-one
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 3-chlorobutan-2-one | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 7 ClO | |||||||||||||||
| Brief description |
clear, colorless to yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 106.55 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.055 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
<−60 ° C |
|||||||||||||||
| boiling point |
129 ° C |
|||||||||||||||
| Vapor pressure |
27.1 hPa (20 ° C) |
|||||||||||||||
| solubility |
28.8 g l −1 (19 ° C) or 35.0 g l −1 (92 ° C) |
|||||||||||||||
| Refractive index |
1.4219 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
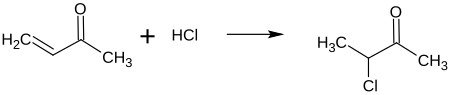
3-chlorobutan-2-one is a chlorinated ketone , it can be understood as a chlorinated and methylated derivative of acetone . 3-chlorobutan-2-one has a stereocenter, so that two enantiomers are present (R) and (S) .
presentation
3-chlorobutan-2-one can be obtained, for example, by chlorinating methyl vinyl ketone with hydrogen chloride:
Individual evidence
- ↑ a b c d e data sheet 3-Chloro-2-butanone, 97% from Sigma-Aldrich , accessed on December 16, 2012 ( PDF ).
- ↑ a b c David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 93rd edition. (Internet version: 2012), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-100. ( Google Books )
- ↑ RM Stephenson: J. Chem. Eng. Data, 37, 80, 1992.
- ↑ Joachim Buddrus: Fundamentals of organic chemistry . 2011, ISBN 978-3-11-024640-7 ( page 644 in the Google book search).