4-chloro-1-naphthol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
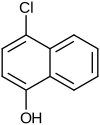
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 4-chloro-1-naphthol | |||||||||||||||
| Molecular formula | C 10 H 7 ClO | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 178.61 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
118-121 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
4-chloro-1-naphthol is a chemical compound that belongs to the group of naphthols .
properties
4-chloro-1-naphthol is used in biochemistry as a chromogenic substrate for immunostaining with a horseradish peroxidase and, after oxidation with hydrogen peroxide in the presence of dimethyl or diethyl analogues of p - phenylenediamine or o -phenylenediamine, produces via the Nadi reaction a blue dye or, in the presence of 3-methyl-2-benzothiazolinonhydrazone, a red dye, e.g. B. ELISA , Western blot and immunohistochemistry .
Individual evidence
- ↑ a b c data sheet 4-chloro-1-naphthol from Sigma-Aldrich , accessed on February 20, 2018 ( PDF ).
- ↑ SM Conyers, DA Kidwell: Chromogenic substrates for horseradish peroxidase. In: Analytical biochemistry. Volume 192, Number 1, January 1991, pp. 207-211, PMID 2048722 .
- ^ J. Uhl, RC Newton: Quantitation of related proteins by Western blot analysis. In: Journal of immunological methods. Volume 110, Number 1, May 1988, pp. 79-84, PMID 3286776 .
- ↑ R. Kobayashi, Y. Tashima: Visualization of antigen on nitrocellulose membrane by the oxidative coupling reaction of N, N′-dimethyl-p-phenylenediamine and 4-chloro-1-naphthol. In: Analytical biochemistry. Volume 183, Number 1, November 1989, pp. 9-12, PMID 2482680 .